��Ŀ����
 ����X��Y��Z��W��R����Ԫ�أ�����ǰ����Ϊ����������Ԫ�أ�Xԭ�ӵ������������Ǻ�����Ӳ�����3����Y���������������۵Ĵ�����Ϊ6��X��Zͬ���壬W��X��Y����ͬ���ڣ�R2+�ļ۵����Ų�ʽΪ3d5��
����X��Y��Z��W��R����Ԫ�أ�����ǰ����Ϊ����������Ԫ�أ�Xԭ�ӵ������������Ǻ�����Ӳ�����3����Y���������������۵Ĵ�����Ϊ6��X��Zͬ���壬W��X��Y����ͬ���ڣ�R2+�ļ۵����Ų�ʽΪ3d5����1��RԪ�������ڱ��е�λ��
��2��X��Y��Z����ͼ��⻯���У��е���ߵ���
��3����R�ľ�����ÿ������ƽ������2��Rԭ�ӣ�����ͼ�о�������?���δ������Rԭ�ӣ�
��4����1.19g ZXY2����100mLˮ�з�����������ԭ��Ӧ������2���ᣬ��Ӧ����ʽΪ
���㣺λ�ýṹ���ʵ����ϵӦ��,�����ļ���
ר�⣺Ԫ����������Ԫ�����ڱ�ר��,��ѧ���뾧��ṹ
������X��Y��Z��W��R����Ԫ�أ�����ǰ����Ϊ����������Ԫ�أ�Xԭ�ӵ������������Ǻ�����Ӳ�����3����ֻ��Ϊ���Ӳ���Ϊ2������������Ϊ6����XΪOԪ�أ�X��Zͬ�壬��ZΪSԪ�أ�Y���������������۴�����Ϊ6�������Ϊ+7�ۣ���YΪClԪ�أ�W��X��Y����ͬ���ڣ���WΪHԪ�أ�R2+�ļ۵����Ų�ʽΪ3d5����R2+��������Ų�Ϊ1s22s22p63s23p63d5��Rԭ�Ӻ��������=2+8+8+5+2=25����RΪMn���ݴ˽��
���
�⣺X��Y��Z��W��R����Ԫ�أ�����ǰ����Ϊ����������Ԫ�أ�Xԭ�ӵ������������Ǻ�����Ӳ�����3����ֻ��Ϊ���Ӳ���Ϊ2������������Ϊ6����XΪOԪ�أ�X��Zͬ�壬��ZΪSԪ�أ�Y���������������۴�����Ϊ6�������Ϊ+7�ۣ���YΪClԪ�أ�W��X��Y����ͬ���ڣ���WΪHԪ�أ�R2+�ļ۵����Ų�ʽΪ3d5����R2+��������Ų�Ϊ1s22s22p63s23p63d5��Rԭ�Ӻ��������=2+8+8+5+2=25����RΪMn��
��1��RΪMnԪ�أ������ڱ��е�λ��Ϊ�������ڢ�B�壻H2O2�ĵ���ʽΪ ��
��
�ʴ�Ϊ���������ڢ�B�壻 ��
��
��2��X��Y��Z����ͼ��⻯���зֱ�ΪH2O��HCl��H2S��ˮ����֮����������������ΪҺ�壬��HCl��H2SΪ��̬����H2O�ķе���ߣ��ǽ�����O��S��Cl��S����H-S���ļ�����С��H2O������Oԭ�Ӽ۲���Ӷ���=2+
=4��Oԭ��Ϊsp3�ӻ���HCl������Clԭ�Ӽ۲���Ӷ���=1+
=4��Clԭ��Ϊsp3�ӻ���H2S������Sԭ�Ӽ۲���Ӷ���=2+
=4��Sԭ��Ϊsp3�ӻ���O��Cl��Sԭ���ӻ�������ͬ��
�ʴ�Ϊ��H2O��H2S����ͬ��
��3����R�ľ�����ÿ������ƽ������2��Rԭ�ӣ�����������1��Rԭ�ӣ����߾�����Rԭ�ӻ�λ�ڶ��㣬��Ӧ����7��Rԭ��λ�ڶ��㣬�����ṹ��ͼ ��
��
�ʴ�Ϊ�� ��
��
��4����1.19g SOCl2����100mLˮ�з�����������ԭ��Ӧ������2���ᣬӦ��ʱHCl��H2SO3����Ӧ����ʽΪSOCl2+2H2O=2HCl+H2SO3��HCl��Ũ��ΪH2SO3��2������H2SO3��������ʣ���Һ��c��Cl-����c��HSO3-����H2SO3��һ������Զ������������룬����Һ��c��HSO3-����c��SO32-������Һ��OH-Ũ����С����������Һ��������Ũ���ɴ�С��˳���ǣ�c��Cl-����c��HSO3-����c��SO32-����c��OH-����
�ʴ�Ϊ��SOCl2+2H2O=2HCl+H2SO3��c��Cl-����c��HSO3-����c��SO32-����c��OH-����
��1��RΪMnԪ�أ������ڱ��е�λ��Ϊ�������ڢ�B�壻H2O2�ĵ���ʽΪ
 ��
���ʴ�Ϊ���������ڢ�B�壻
 ��
����2��X��Y��Z����ͼ��⻯���зֱ�ΪH2O��HCl��H2S��ˮ����֮����������������ΪҺ�壬��HCl��H2SΪ��̬����H2O�ķе���ߣ��ǽ�����O��S��Cl��S����H-S���ļ�����С��H2O������Oԭ�Ӽ۲���Ӷ���=2+
| 6-1��2 |
| 2 |
| 7-1��1 |
| 2 |
| 6-1��2 |
| 2 |
�ʴ�Ϊ��H2O��H2S����ͬ��
��3����R�ľ�����ÿ������ƽ������2��Rԭ�ӣ�����������1��Rԭ�ӣ����߾�����Rԭ�ӻ�λ�ڶ��㣬��Ӧ����7��Rԭ��λ�ڶ��㣬�����ṹ��ͼ
 ��
���ʴ�Ϊ��
 ��
����4����1.19g SOCl2����100mLˮ�з�����������ԭ��Ӧ������2���ᣬӦ��ʱHCl��H2SO3����Ӧ����ʽΪSOCl2+2H2O=2HCl+H2SO3��HCl��Ũ��ΪH2SO3��2������H2SO3��������ʣ���Һ��c��Cl-����c��HSO3-����H2SO3��һ������Զ������������룬����Һ��c��HSO3-����c��SO32-������Һ��OH-Ũ����С����������Һ��������Ũ���ɴ�С��˳���ǣ�c��Cl-����c��HSO3-����c��SO32-����c��OH-����
�ʴ�Ϊ��SOCl2+2H2O=2HCl+H2SO3��c��Cl-����c��HSO3-����c��SO32-����c��OH-����
���������⿼��ṹ����λ�ù�ϵӦ�ã��漰��������Ų�������ʽ�����ӽṹ�����ʡ���ѧ�����ӻ����ۡ������ṹ������Ũ�ȱȽϵȣ���Ŀ�Ƚ��ۺϣ���Ҫѧ���������ջ���֪ʶ���Ѷ��еȣ��ƶ�Ԫ���ǽ���ؼ���

��ϰ��ϵ�д�
 ѧ���쳵�����ּ��ں�����ҵϵ�д�
ѧ���쳵�����ּ��ں�����ҵϵ�д� ��˼ά������ҵϵ�д�
��˼ά������ҵϵ�д�
�����Ŀ
��ŵ���ǽ�����ʹ����ҩ����ṹ��ͼ��ʾ�������й�˵����ȷ���ǣ�������


| A������ʽΪC17H14O5N |
| B������������ˮ��Һ��ˮ����Եõ�3���л������� |
| C��1mol��ŵ����H2�����ӳɷ�Ӧ�������8mol H2 |
| D����ŵ�����������������ڵ������� |
����˵����ȷ���ǣ�������
| A�������£���Ӧ4Fe��OH��2��s��+2H2O��l��+O2��g���T4Fe��OH��3��s�����Է����У����H��0 | ||
| B����Ƭ��пʱ����Ƭ�����Դ���������� | ||
| C��NaClO��Һ��ͨ������CO2��ClO-ˮ��̶�������Һ������ǿ | ||
D��t��ʱ�������ܱ������з�Ӧ��NO2��g��+SO2��g��?NO��g��+SO3��g����ͨ������O2��
|
��ù����һԪ�л��ᣬ�������ε�1���ʵ�λ������Ϊ6.00��10-7�ˣ����ļ���1���ʵ�λ������Ϊ6.27��10-7�ˣ���1���ʵ�λ��2���ε����ʵ�����ȣ�������ù�ص���Է�������Ϊ��������
| A��371.6 |
| B��355.6 |
| C��333.6 |
| D��332.6 |
����ʵ�鷽���ܴﵽʵ��Ŀ���ǣ�������
| ��� | A | B | C | D |
| ʵ�� ���� |
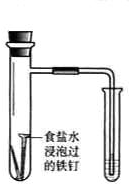 ʳ��ˮ |
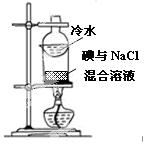 |
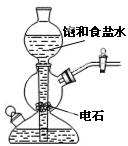 |
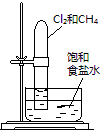 ���ڹ����� |
| ʵ�� Ŀ�� |
��֤�����������ⸯʴ | �ӵ���NaCl�����Һ�з������ | ʵ�����Ʊ���Ȳ | ��֤����������������ѧ��Ӧ |
| A��A | B��B | C��C | D��D |
 һ�������£�����[CO��NH2��2]��NO��Ӧ����N2������2�ֶԻ�������Ⱦ���������ͼ��CO��NH2��2��ij��������NO�����ʵ���֮�ȷֱ�Ϊ1��2��2��1��3��1ʱ��NO�ѳ������¶ȣ�t���仯��ͼ������˵��������ǣ�������
һ�������£�����[CO��NH2��2]��NO��Ӧ����N2������2�ֶԻ�������Ⱦ���������ͼ��CO��NH2��2��ij��������NO�����ʵ���֮�ȷֱ�Ϊ1��2��2��1��3��1ʱ��NO�ѳ������¶ȣ�t���仯��ͼ������˵��������ǣ�������| A�������ѳ�NO�ķ�ӦΪ��2CO��NH2��2+6NO�T2CO2+4H2O+5N2 |
| B��ͼ��a����ӦCO��NH2��2��NO�����ʵ���֮��Ϊ1��2 |
| C����900�����ң�NO���ѳ������ |
| D��NO����ʼŨ��Ϊ6��10-4mg/m3��ͼ��a��A�㵽B�㾭��0.8s�����ʱ����NO���ѳ�ƽ������Ϊ1.5��10-4mg/��m3?s�� |


 �����£�N2H4���ǵ������ֳ����⻯��ڿ�ѧ����������������Ҫ��Ӧ�ã�
�����£�N2H4���ǵ������ֳ����⻯��ڿ�ѧ����������������Ҫ��Ӧ�ã� ͨ����ˮ�ܻ�õ�ˮ��ʳ�Ρ�þ�ȣ�ʳ�οɽ�һ�������ȼҵ����ش��������⣮
ͨ����ˮ�ܻ�õ�ˮ��ʳ�Ρ�þ�ȣ�ʳ�οɽ�һ�������ȼҵ����ش��������⣮