��Ŀ����
����������ȷ���ǣ�������
| A��ijҺ�����ʵ�Ħ����������18 g?mol-1����������ܶ�һ����ˮ�� |
| B��c mol?L-1 KCl��Һ���ܶ�Ϊ��g/cm3�����KCl��Һ����������Ϊ74.5c��1000�ѡ�100% |
| C����90% H2SO4��Һ��10% H2SO4��Һ�������ϣ�����������һ������50% |
| D��ͬ��ͬѹ�£��ֱ���HCl��NO2����֧�Թܵ���������ˮ���㹻��ʱ�䣬���費����������ɢ����֧�Թ�����Һ�����ʵ���Ũ��֮��Ϊ3��2 |
���㣺Ħ������,��Һ�����ʵ�������������ؼ���
ר�⣺������
������A������ͬ�����£�����ı仯��ֱ��Ӱ���ܶȣ�
B�������������������ʵ���Ũ�Ȼ��㷽����������жϣ�
C����90% H2SO4��Һ��10% H2SO4��Һ�������ϣ���Һ�ܶȴ���1������������һ������50%��
D��ʢ�е�����Ȼ�����Թܵ�����ˮ����ʱ��ˮ�����������ƿ������������ˮ��Ӧ����ʽΪ��3NO2+2H2O=2HNO3+NO�����ݷ���ʽ֪��ˮ�������ƿ��
������C=
������Һ�����ʵ���Ũ�ȣ�
B�������������������ʵ���Ũ�Ȼ��㷽����������жϣ�
C����90% H2SO4��Һ��10% H2SO4��Һ�������ϣ���Һ�ܶȴ���1������������һ������50%��
D��ʢ�е�����Ȼ�����Թܵ�����ˮ����ʱ��ˮ�����������ƿ������������ˮ��Ӧ����ʽΪ��3NO2+2H2O=2HNO3+NO�����ݷ���ʽ֪��ˮ�������ƿ��
| 2 |
| 3 |
| n |
| V |
���
�⣺A��ijҺ�����ʵ�Ħ����������18 g?mol-1������ͬ�����£�����ı仯��ֱ��Ӱ���ܶȣ��������ܶȲ�һ����ˮ�����Ҵ�Ħ������Ϊ46g/mol�����ܶ�С��1����A����
B��c mol?L-1 KCl��Һ���ܶ�Ϊ��g/cm3�����KCl��Һ����������=
��100%����B��ȷ��
C����90% H2SO4��Һ��10% H2SO4��Һ��������ϣ��������������غ㣬����������һ������50%�������ܶȴ���1����90% H2SO4��Һ��10% H2SO4��Һ�������ϣ�����������һ������50%����C����
D����ͬ�����£�����������������ʵ�����ȣ��Ȼ��⼫������ˮ����ʢ���Ȼ�����Թܵ�����ˮ����ʱ��ˮ�����������ƿ��
����������ˮ��Ӧ����ʽΪ��3NO2+2H2O=2HNO3+NO�����ݷ���ʽ֪��ˮ�������ƿ��
����Һ�е����������ᣬ�����ʵ����Ƕ���������
��
������ƿ�������1L����n��HCl��=n��NO2��=
��
��Һ�����ʵ����ʵ����ֱ��ǣ�n��HCl��=
��n��HNO3��=
��
��
��Һ������ֱ���V��HCl��=1L��V��HNO3��=
��
����C=
֪����Һ�����ʵ���Ũ����ȣ�������Ũ��֮��Ϊ1��1����D����
��ѡB��
B��c mol?L-1 KCl��Һ���ܶ�Ϊ��g/cm3�����KCl��Һ����������=
| 74.5c |
| 1000���� |
C����90% H2SO4��Һ��10% H2SO4��Һ��������ϣ��������������غ㣬����������һ������50%�������ܶȴ���1����90% H2SO4��Һ��10% H2SO4��Һ�������ϣ�����������һ������50%����C����
D����ͬ�����£�����������������ʵ�����ȣ��Ȼ��⼫������ˮ����ʢ���Ȼ�����Թܵ�����ˮ����ʱ��ˮ�����������ƿ��
����������ˮ��Ӧ����ʽΪ��3NO2+2H2O=2HNO3+NO�����ݷ���ʽ֪��ˮ�������ƿ��
| 2 |
| 3 |
| 2 |
| 3 |
������ƿ�������1L����n��HCl��=n��NO2��=
| 1 |
| 22.4 |
��Һ�����ʵ����ʵ����ֱ��ǣ�n��HCl��=
| 1 |
| 22.4 |
| 1 |
| 22.4 |
| 2 |
| 3 |
��Һ������ֱ���V��HCl��=1L��V��HNO3��=
| 2 |
| 3 |
����C=
| n |
| V |
��ѡB��
���������⿼������Һ�ܶȴ�С�Ƚϣ��������������ʵ���Ũ�Ȼ��㣬ע����������ˮ���γ���ҺŨ�ȵļ��㷽������Ŀ�ѶȽϴ�

��ϰ��ϵ�д�
 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д�
�����Ŀ
������Һ����CH3COOH ��HCl ��NaOH ��CH3COONa ��KCl ��NH4Cl�����ʵ���Ũ�Ⱦ�Ϊ0.1mol/L����pH��С��������˳��Ϊ��������
| A���ۢܢݢޢ٢� |
| B���ܢݢޢ٢ۢ� |
| C���ڢ٢ޢݢܢ� |
| D���ڢۢ٢ޢݢ� |
���и�������������ķ�����һ����ȵ��ǣ�������
| A���¶���ͬ�������ͬ��O2��N2 |
| B��������ȡ��ܶȲ��ȵ�N2��C2H4 |
| C�������ͬ���ܶ���ȵ�CO��C2H4 |
| D��ѹǿ��ͬ�������ͬ��O2��H2 |
���л�ѧʵ��������¹ʴ���������ȷ���ǣ�������
| A��ʵ�������ֱ�����촵��ƾ��� |
| B��Ũ����մ��Ƥ����ʱ��������ʪĨ����ϴ��Ȼ��Ϳ��3%��5%��NaOH��Һ |
| C����Һʱ����Һ©�����²�Һ����¿ڷų����ϲ�Һ����Ͽڵ��� |
| D������������Һʱ����������Ͳ�м���һ�������ˮ�����ڽ�����������������Ũ���� |
���й���1.0mol?L-1��NaCl��Һ��˵����ȷ���ǣ�������
| A����Һ�к���1mol NaCl |
| B��1L��Һ�к���58.5g NaCl |
| C��1mol NaCl����1Lˮ�� |
| D��58.5g NaCl����941.5gˮ�� |
���й���ʳƷ���Ӽ��������в���ȷ���ǣ�������
| A����ͬʱ�����������͵�ζ�� |
| B���������ƿ��������������������˹��� |
| C���յ��죨����Ϊ��ɫ�� |
| D���������ƿ�����ѩ�����ϵķ����� |
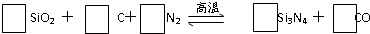
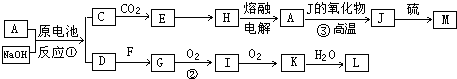 A��J���ճ������г��������ֽ����������ֽ�����NaOH���ԭ��أ�A��������F�����������嵥�ʣ������������µ�ת����ϵ�����ֲ��P������ȥ����
A��J���ճ������г��������ֽ����������ֽ�����NaOH���ԭ��أ�A��������F�����������嵥�ʣ������������µ�ת����ϵ�����ֲ��P������ȥ����