��Ŀ����
ʵ������50mL0.50mol?L-1���ᡢ50mL0.55mol?L-1NaOH��Һ����ͼ��ʾװ�ý��вⶨ���ȵ�ʵ�飬�õ����е����ݣ�
������������⣺
��1��ʵ��ʱ�û��β��������������Һ�ķ����� ��������ͭ˿��������沣������������ ��
��2�������ݴ�����t2-t1=3.4�棬���ʵ���õ��к��ȡ�H= ��[�����NaOH��Һ���ܶȰ�1g?cm-3���㣬��Ӧ������Һ�ı����ݣ�c����4.18J?��g?�棩-1����]��
��3������50mL0.50mol?L-1 NaOH��Һ��������ʵ�飬������к���Ϊ��H1�����H1 ��H�����������������=����
| ʵ����� | ��ʼ�¶�t1/�� | ��ֹ�¶� t2/�� | |
| ���� | NaOH | ||
| 1 | 20.2 | 20.3 | 23.7 |
| 2 | 20.3 | 20.5 | 23.8 |
| 3 | 21.5 | 21.6 | 24.9 |
��1��ʵ��ʱ�û��β��������������Һ�ķ�����
��2�������ݴ�����t2-t1=3.4�棬���ʵ���õ��к��ȡ�H=
��3������50mL0.50mol?L-1 NaOH��Һ��������ʵ�飬������к���Ϊ��H1�����H1
���㣺�к��ȵIJⶨ
ר�⣺ʵ����
��������1�������к��ȵIJⶨʵ���л��β���������IJ���������𣻽������ȿ죬������ʧ�ࣻ
��2����������η�Ӧ���¶Ȳ���ݹ�ʽQ=cm��T���������0.05mol��ˮ�ų��������������к��ȵĸ��������Ӧ�ȣ�
��3����Ӧ�ų����������������Լ�������Ķ����йأ��������к��ȵĸ����ʵ�����ش�
��2����������η�Ӧ���¶Ȳ���ݹ�ʽQ=cm��T���������0.05mol��ˮ�ų��������������к��ȵĸ��������Ӧ�ȣ�
��3����Ӧ�ų����������������Լ�������Ķ����йأ��������к��ȵĸ����ʵ�����ش�
���
�⣺��1��ʵ��ʱ�û��β��������������Һ�ķ��������³鶯��ͭ˿��������ȵ������壬������ʧ�����������Բ��ܽ����β����������Ϊͭ˿�������
�ʴ�Ϊ�����³鶯���������ȿ죬������ʧ��������
��2��50mL 0.50mol?L-1���ᡢ50mL 0.55mol?L-1 NaOH��������Ϊm=100mL��1g/mL=100g��c=4.18J/��g?�棩����T=t2-t1=3.4�棬���빫ʽQ=cm��T������0.025mol��ˮ�ų�����Q=4.18J/��g?�棩��100g��3.4��=1421.2J=1.4212KJ��������0.025mol��ˮ�ų�����1.4212KJ����������1mol��ˮ�ų�����Ϊ
=56.8kJ������ʵ���õ��к��ȡ�H=-56.8kJ/mol��
�ʴ�Ϊ��-56.8kJ/mol��
��3����Ӧ�ų����������������Լ�������Ķ����йأ������к��ȵľ���ǿ���ǿ�Ӧ����1molˮʱ�ų����ȣ������������أ�����50mL0.50mol?L-1 NaOH��Һ��������ʵ�飬������к���Ϊ��H1�����H1=��H��
�ʴ�Ϊ��=��
�ʴ�Ϊ�����³鶯���������ȿ죬������ʧ��������
��2��50mL 0.50mol?L-1���ᡢ50mL 0.55mol?L-1 NaOH��������Ϊm=100mL��1g/mL=100g��c=4.18J/��g?�棩����T=t2-t1=3.4�棬���빫ʽQ=cm��T������0.025mol��ˮ�ų�����Q=4.18J/��g?�棩��100g��3.4��=1421.2J=1.4212KJ��������0.025mol��ˮ�ų�����1.4212KJ����������1mol��ˮ�ų�����Ϊ
| 1.4212KJ��1mol |
| 0.025mol |
�ʴ�Ϊ��-56.8kJ/mol��
��3����Ӧ�ų����������������Լ�������Ķ����йأ������к��ȵľ���ǿ���ǿ�Ӧ����1molˮʱ�ų����ȣ������������أ�����50mL0.50mol?L-1 NaOH��Һ��������ʵ�飬������к���Ϊ��H1�����H1=��H��
�ʴ�Ϊ��=��
���������⿼���к��ȵIJⶨ����Ŀ�ѶȲ���ע���������㹫ʽ��Ӧ����c=4.18J/��g?�棩��Ҫע��������λ�Ļ��㣮

��ϰ��ϵ�д�
�����Ŀ
 ������ֳƻƼ���ҹ��ض�����ҩ�������������һ���������ҹ���ѧ�������о�������ṹ��ͼ��
������ֳƻƼ���ҹ��ض�����ҩ�������������һ���������ҹ���ѧ�������о�������ṹ��ͼ��������к��������ŵ�������
�����л���Ӧ�У�������ȡ����Ӧ���ǣ�������
A��CH3COOH+CH3CH2OH
| |||
B��2CH3CH2OH
| |||
C��CH3CH2OH
| |||
D��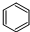 +Br2 +Br2
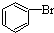 +HBr +HBr |
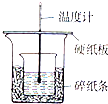 ʵ����������ͼװ�ý����к��ȵIJⶨ����ش��������⣺
ʵ����������ͼװ�ý����к��ȵIJⶨ����ش��������⣺