��Ŀ����
13��CoCl2•6H2O��һ������Ӫ��ǿ�������Ժ��ܷ��ϣ�������Fe��Al�����ʣ���ȡCoCl2•6H2O��һ���¹���������ͼ��
��֪��
���������ᷴӦ�Ļ�ѧ����ʽΪ��Co+2HCl��CoCl2+H2��
��CoCl2•6H2O�۵�86�棬������ˮ�����ѵȣ��������ȶ�����������110��120��ʱ��ʧȥ�ᾧˮ����ж�����ˮ�Ȼ��ܣ�
�۲���������������������ʽ����ʱ��Һ��pH������
| ������ | Fe��OH��3 | Fe��OH��2 | Co��OH��2 | Al��OH��3 |
| ��ʼ���� | 2.3 | 7.5 | 7.6 | 3.4 |
| ��ȫ���� | 4.1 | 9.7 | 9.2 | 5.2 |
��2������̼���Ƶ���pH��a��a�ķ�Χ��5.2��7.6��
��3�����������3��ʵ�������������������������Ũ������ȴ�ᾧ���ˣ�
��4���Ƶõ�CoCl2•6H2O�ں��ʱ���ѹ��ɵ�ԭ���ǽ��ͺ���¶ȣ���ֹ��Ʒ�ֽ⣮
��5��Ϊ�ⶨ��Ʒ��CoCl2•6H2O������ijͬѧ��һ��������Ʒ����ˮ���������м���������AgNO3��Һ�����ˣ�����������ɺ������������ͨ�����㷢�ֲ�Ʒ��CoCl2•6H2O��������������100%����ԭ���������Ʒ�к���NaCl���ʣ�CoCl2•6H2O���ʱʧȥ�˲��ֽᾧˮ��
��6����ʵ���ң�Ϊ�˴�������Ʒ�л�ô�����CoCl2•6H2O��ͨ���Ƚ���Ʒ�ܽ��������У���ȥ���������ʺ��ٽ������������
���� ���ܷ����м������ᣬ�ɵ�CoCl2��AlCl3��FeCl2������������⣬�ɵõ�FeCl3��Ȼ�����Na2CO3��pH��5.2���ɵõ�Fe��OH��3��Al��OH��3���������˺�������Һ��Ҫ����CoCl2��Ϊ�õ�CoCl2•6H2O���壬Ӧ�����¶���86�����£�����ʱҪ��ֹ�¶ȹ��߶�ʧȥ�ᾧˮ���ɼ�ѹ��ɣ��Դ˽����⣮
��1�����������ᷴӦ�����Ȼ�������ᷴӦ���������Σ�ͬʱ����һ��������
��2��������Һ��PHֵ��ʹ��Һ�������Ӻ����������ɳ������������Ӵ�����Һ�У��Ӷ��������ӡ������Ӻ������ӷֿ���
��3�����ݴ���Һ����ȡ����ķ�����ȡ�Ȼ��ܹ��壬�Ӷ�ȷ���������裻
��4���¶ȸ�ʱ��CoCl2•6H2O�ֽ⣻
��5������CoCl2•6H2O����ɷ�������ɲ�Ʒ��CoCl2•6H2O��������������100%��ԭ������ǣ�1���������ʣ����������Ӻ�����2���ᾧˮ����ʧȥ����ˮ��
��6������CoCl2•6H2O������ѡȡ������CoCl2•6H2o�۵�86�棬���������ѣ�
��� �⣺��1�����������ᷴӦ�����Ȼ�������ᷴӦ���������Σ�ͬʱ����һ�������������Ȼ����к������������ʣ��������ж����壬��Ⱦ������
�ʴ�Ϊ�������ж�������ŷţ���ֹ������Ⱦ����ֹ��Ʒ�л��������Σ�
��2�����ϼ�������������Ȼ��Ȼ�����˫��ˮ�����������ӱ�˫��ˮ�����������������ӣ����ݳ�����PHֵ����֪������Һ��PHֵΪ5.2ʱ�������Ӻ������ӱ���ȫ����������Һ��PHֵΪ7.6ʱ�������Ӳſ�ʼ��������������Ҫ�뽫�����ӡ������Ӻ������ӷ��룬��Һ��pHֵӦ�ò�С��5.2������7.6��pH����a����ˣ������Լ�X����Һ��pH������2��3�����õ��Լ�XΪ���
�ʴ�Ϊ��5.2��7.6��
��3������Һ����ȡ����ķ�����ȡ�Ȼ��ܹ��壬���������������Ũ������ȴ�ᾧ���ˣ�
�ʴ�Ϊ������Ũ������ȴ�ᾧ��
��4����������֪��CoCl2•6H2O�������ȶ�����������110��120��ʱ��ʧȥ�ᾧˮ����ж�����ˮ�Ȼ��ܣ�Ϊ��ֹ��ֽ⣬�Ƶõ�CoCl2•6H2O���ѹ��ɣ�
�ʴ�Ϊ�����ͺ���¶ȣ���ֹ��Ʒ�ֽ⣻
��5������CoCl2•6H2O����ɷ�������ɲ�Ʒ��CoCl2•6H2O��������������100%��ԭ������ǣ�1���������ʣ����������Ӻ�����2���ᾧˮ����ʧȥ����ˮ��������ͬ�����Ĺ����������Ӻ������
�ʴ�Ϊ����Ʒ�к���NaCl���ʣ�CoCl2•6H2O���ʱʧȥ�˲��ֽᾧˮ��
��6������CoCl2•6H2O������ѡȡ������CoCl2•6H2O�۵�86�棬���������ѣ����Խ���Ʒ�������ѣ����˺������Ӷ���ýϴ�����CoCl2•6H2O��
�ʴ�Ϊ������
���� ���⿼����ʵ�鷽������е��й�֪ʶ���ѶȽϴȽ�ͻ�����ѵ��ǣ�1�����ķ�����֪���Ѱ�ҡ����ۡ���2����Ϣ������Ϣ���Ķ�����ȡ������ϢȻ����мӹ��ķ������ᣬ�Ӷ������������϶࣮

 ˮ�����������һ����Ҫ���л��ϳ�ԭ�ϣ�ij��ѧС����ˮ���ᣨ�ṹ��ʽΪ
ˮ�����������һ����Ҫ���л��ϳ�ԭ�ϣ�ij��ѧС����ˮ���ᣨ�ṹ��ʽΪ ���ͼ״������Դ������ºϳ�ˮ�����������������ʣ�
���ͼ״������Դ������ºϳ�ˮ�����������������ʣ�ʵ�鲽�裺
����ͼ����������ƿ�м���13.8g ��0.1mol��ˮ�����24g��30mL��0.75mol���״����������м���Լ10mL�ױ����ױ���ˮ�γɵĹ�����е�Ϊ85�棬��ʵ���м���ױ�����ˮ����������С�ĵؼ���5mLŨ���ᣬҡ�����ȣ�����1��2����ʯ����װ��ʵ��װ�ã���85��95���º��¼��ȷ�Ӧ1.5Сʱ��
��װ����ȴ������״���Ȼ��ת������Һ©��������������ˮ��5%NaHCO3��Һ��ˮϴ�ӣ��ֳ��IJ������������ˮMgSO4���壬���˵õ�������
���������������ռ�221�桫224�����֣���ˮ�������9.12g��
��������������
| ���� | ������ | ��ɫ״̬ | ����ܶ� | �۵㣨�棩 | �е㣨�棩 |
| ˮ������� | 152 | ��ɫҺ�� | 1.18 | -8.6 | 224 |
| ˮ���� | 138 | ��ɫ���� | 1.44 | 158 | 210 |
| �״� | 32 | ��ɫҺ�� | 0.792 | -97 | 64.7 |
��1������A�����������������ܣ�
��2��ʵ���м���ױ��Ժϳ�ˮ��������������Ƿ�Ӧ������ˮ�ӷ�Ӧ��ϵ�з��뿪����ʹ��ƽ�������ƶ���ͬʱ���Լ��ټ״����������Ӷ���߷�Ӧ�IJ��ʣ�
��3��ʵ���м�����ˮ����þ�������Ǹ������
��4����Ӧ����������״����õķ�����������������ƣ���
��5����ʵ��IJ���Ϊ60%��
| �۵�/�� | �е�/�� | |
| 1-���� | -89.53 | 117.25 |
| 1-�嶡�� | -112.4 | 101.6 |
| ���� | -95.3 | 142.4 |
| 1-��ϩ | -185.3 | -6.5 |
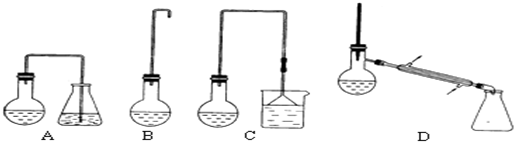
��1���Ʊ�1-�嶡���װ��Ӧѡ����ͼ�е�C������ţ�����Ӧ����ʱ���¶Ȳ��˳���100�棬�����Ƿ�ֹ1-�嶡�����������ݳ���Ӱ��������¶�̫�ߣ�Ũ������������ǿ�����������廯�⣮
��2���Ʊ������У������Ũ������廯�Ƶ������Ƕ��߷�Ӧ����HBr��
��3����Ӧ��������Ӧ�������1-�嶡����������Ӧѡ�õ�װ����D��������ţ����ò���Ӧ���Ƶ��¶ȣ�t����Χ��101.6���t��117.25�森
��4������ȥ������е���������Br2���������������ʺϵ���c��������ĸ��
a��NaI b��NaOH c��Na2SO3 d��KCl��
��1��ʵ�����Ʊ�FeCO3���������£�

��д�����ɳ����Ļ�ѧ����ʽ��FeSO4+2NH4HCO3=FeCO3��+��NH4��2SO4+CO2��+H2O��
�ڼ�����Һ�к���NH4+�ķ�����ȡ������Һ���Թ��У���������NaOH��Һ�����ȣ������������ʹʪ��ĺ�ɫʯ����ֽ����������Һ�к���NH4+��
��2����ͬѧΪ����֤FeCO3�����������Ƿ�õ�Fe2O3���������ʵ�飺
| ʵ�鲽�� | ʵ��������� |
| �� | ȡһ��������FeCO3�������������У������������������ټ��ᣬ��ȴ������ |
| �� | ȡ����ʵ�鲽������ù�������һ�ྻ���Թ��У���������ϡ�����ܽ� |
| �� | ��ʵ�鲽���������Һ�еμ�KSCN��Һ����Һ��� |
��ʵ�鲽�������Һ�������ӷ���ʽΪFe3++3SCN-=Fe��SCN��3��
����ͬѧ����˲�ͬ�Ŀ��������ղ��������Fe3O4����ΪFe3O4Ҳ�����������ᣬ��������Һ��Ҳ����Fe3+��������ͬѧ��ʵ�鲽�������˲��䣺��ȡ����ʵ�鲽���������Һ��Ȼ��D������ĸ��������������Һ���Ƿ���Fe2+��
A���μ���ˮB���μ�KSCN��Һ
C���ȵμ�KSCN��Һ��μ���ˮD���μ�����KMnO4��Һ
��3����ͬѧ��Ϊ��ʹ�õ���ͬѧԤ�ڵ�ʵ������Ҳ����ȷ�����ղ��TΪFe3O4��
�ٱ�ͬѧ�ִ˿���������������������������������Ҳ�Ǻ���+2����Ԫ�صģ�
�ڱ�ͬѧ�������ϵ�֪����FeCO3�IJ����к���+2����Ԫ�أ��������������FeCO3�Ʊ�Fe2O3�ķ���������FeCO3��ĩ�����μ����Լ���ϡ���ᡢ��ˮ���������ƣ�Ȼ���ٹ��ˣ����������ϴ�ӡ����ռ��ɵõ�Fe2O3��
ijʵ��С����������к͵ζ����ⶨʳ����������CH3COOH��g/100mL����
��һ����ʵ����Ʒ������ʳ�ð״���Ʒ500mL���̱�ע������������3.5g-5g/100mL�����³ơ�ԭ�ס�����0.1000mol/LNaOH����Һ������ˮ��0.1%������Һ��0.1%��̪��Һ��������ѡ��
��������ʵ��ԭ������ѧ����ʽCH3COOH+NaOH=CH3COONa+H2O
��������ʵ�鲽�裺
��1�����ƴ���ʳ����Һ
ȷ��ȡ10.00mLԭ�ף����Ƴ�100.00mL����ʳ����Һ���³ơ�����Һ��������ɸò��裬��Ҫ��һ�ֶ���������100ml����ƿ�����������ƣ�
��2����ȡһ������Ĵ���Һ
����ʽ�ζ���ȡ20.00mL����Һ����ƿ�У���1-2��0.1%��̪��Һ��ָʾ��
��3��ʢװ��NaOH��Һ����¼ΪNaOH����Һ����ij�������
��4���ζ��������ζ�ʱһ�ֿ��Ƶζ��ܣ�һ��ҡ����ƿ���۾��۲���ƿ����Һ��ɫ�仯ֱ���ζ��յ㣮�жϵζ��յ������������ɫ��Ϊdz��ɫ��������ڲ���ɫ��
��¼NaOH���ն������ظ��ζ�4�Σ�
���ģ���ʵ���¼�����ݴ���������������գ�
| �ζ����� ʵ������ | 1 | 2 | 3 | 4 |
| V������Һ��/mL | 20.00 | 20.00 | 20.00 | 20.00 |
| V��NaOH��/mL���������� | 0.00 | 0.200 | 0.10 | 0.00 |
| V��NaOH��/mL���ն����� | 14.95 | 15.20 | 15.15 | 16.95 |
| V��NaOH��/mL�����ģ� | 14.95 | 15.00 | 15.05 | 16.95 |
��6������NaOH��Һ��ƽ��ֵ��15.00mL��
��7������Һ��Ũ����0.075mol/L��
 ij��ѧʵ��С����Ҫ�˽��г�������ʳ�ð״ף���Ҫ�Ǵ����ˮ��Һ����ȷŨ�ȣ��ִ��г�������һƿijƷ��ʳ�ð״ף���ʵ�������ñ�NaOH��Һ������еζ����±���4�ֳ���ָʾ���ı�ɫ��Χ��
ij��ѧʵ��С����Ҫ�˽��г�������ʳ�ð״ף���Ҫ�Ǵ����ˮ��Һ����ȷŨ�ȣ��ִ��г�������һƿijƷ��ʳ�ð״ף���ʵ�������ñ�NaOH��Һ������еζ����±���4�ֳ���ָʾ���ı�ɫ��Χ��| ָʾ�� | ʯ�� | ���� | ���� | ��̪ |
| ��ɫ��Χ��pH�� | 5.0��8.0 | 3.1��4.4 | 4.4��6.2 | 8.2��10.0 |
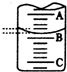
��2����ͼ��ʾ50mL�ζ�����Һ���λ�ã���A��C�̶ȼ����1mL��A���Ŀ̶�Ϊ25���ζ�����Һ�����ӦΪ25.40mL����ʱ�ζ�����Һ����� ������24.60ml��
��3��Ϊ�˼�Сʵ������ͬѧһ������������ʵ�飬����ÿ����ȡ�״������ΪVmL��NaOH��ҺŨ��Ϊc mo1/L������ʵ������¼���£�
| ʵ����� | ��һ�� | �ڶ��� | ������ |
| ����NaOH��Һ���/mL | 26.02 | 25.35 | 25.30 |
A��ʵ�����ʱ���ӵζ�����Һ�棬��ȡ�ζ��յ�ʱNaOH��Һ�����
B���μ�NaOH��Һ���죬δ������տ�����Һ��ɫ������ֹͣ�ζ�
C��ʢװ��Һ�ĵζ���װҺǰ������ˮϴ�ӣ�δ�ñ�Һ��ϴ
D����һ�εζ��õ���ƿ�ô�װҺ��ϴ����������δ��ϴ
��4�������������ݣ�д������ð״��д�������ʵ���Ũ�ȵı���ʽ�����ػ���c��CH3COOH��=$\frac{c����25.35+25.30��}{2V}$mol/L��
| A�� | ���ȷ�Ӧ�ڳ����¶��������� | |
| B�� | ���ȷ�Ӧ�����ȾͲ��ᷢ�� | |
| C�� | ��Ҫ���Ȳ��ܷ����ķ�Ӧ�������ȷ�Ӧ | |
| D�� | ���ݷ�Ӧ����������������������Դ�С��ȷ����Ӧ�� |

