��Ŀ����
��֪���ᾧ�壨H2C2O4��XH2O��������ˮ�����������Ը��������Һ��ȫ��Ӧ��2KMnO4+5H2C2O4+3H2SO4=K2SO4+2MnSO4+8H2O+10CO2��
����������ԭ�ζ����ⶨ���ᾧ��Ľᾧˮ������X���������£�
���÷�����ƽ��ȡ���ᾧ��1.260g���������Ƴ�100.00mL���������Һ
������Һ����ȡ25.00mL���������Һ����ƿ�У����������������ữ
����Ũ��Ϊ0.1000mol��L-1��KMnO4����Һ���еζ������ν�����£�
��֪H2C2O4����Է�������Ϊ90����ش��������⣺
��1���ζ�ʱ��KMnO4����ҺӦ��װ�� ������ʽ���ʽ���ζ����У�
��2��������ʵ������У�����Ҫ����������Ʒ�� ������ţ���
��100mL����ƿ ���ձ� �۵ζ��ܼ� ��©�� �ݲ����� ��������ƽ
��3������ζ��յ�ı�־�� ��
��4�������������ݼ���X= ��
��5�����������ƫ�ߡ�ƫ�͡���Ӱ�죩��
�����ζ���ʼʱ���ӵζ��̶ܿȣ��ζ�����ʱ���ӵζ��̶ܿȣ���Xֵ ��
����KMnO4����ҺŨ��ƫ�ͣ���Xֵ ��
����������ԭ�ζ����ⶨ���ᾧ��Ľᾧˮ������X���������£�
���÷�����ƽ��ȡ���ᾧ��1.260g���������Ƴ�100.00mL���������Һ
������Һ����ȡ25.00mL���������Һ����ƿ�У����������������ữ
����Ũ��Ϊ0.1000mol��L-1��KMnO4����Һ���еζ������ν�����£�
| ��һ�εζ� | �ڶ��εζ� | �����εζ� | |
| ������Һ�����mL�� | 25.00 | 25.00 | 25.00 |
| ����Һ�����mL�� | 9.99 | 10.01 | 10.60 |
��1���ζ�ʱ��KMnO4����ҺӦ��װ��
��2��������ʵ������У�����Ҫ����������Ʒ��
��100mL����ƿ ���ձ� �۵ζ��ܼ� ��©�� �ݲ����� ��������ƽ
��3������ζ��յ�ı�־��
��4�������������ݼ���X=
��5�����������ƫ�ߡ�ƫ�͡���Ӱ�죩��
�����ζ���ʼʱ���ӵζ��̶ܿȣ��ζ�����ʱ���ӵζ��̶ܿȣ���Xֵ
����KMnO4����ҺŨ��ƫ�ͣ���Xֵ
���㣺�к͵ζ�
ר�⣺ʵ����
��������1��KMnO4��Һ����ǿ�����ԣ����Ը�ʴ��Ƥ�ܣ�
��2������һ�����ʵ���Ũ�ȵ���Һ��Ҫ����ƿ���ձ������������ζ�������Ҫ�ζ��ܼУ������÷�����ƽ��
��3������KMnO4��Һ��������ɫ��Ϊָʾ���жϵζ��յ�ʱ���ٵμ�KMnO4��Һʱ����Һ������ɫ��Ϊdz�Ϻ�ɫ��
��4�������к͵ζ��������������ʵ������Ӷ�����X��ֵ��
��5����c���⣩=
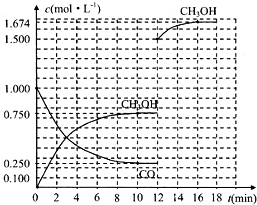
���������������V���ζ����ı仯������
��2������һ�����ʵ���Ũ�ȵ���Һ��Ҫ����ƿ���ձ������������ζ�������Ҫ�ζ��ܼУ������÷�����ƽ��
��3������KMnO4��Һ��������ɫ��Ϊָʾ���жϵζ��յ�ʱ���ٵμ�KMnO4��Һʱ����Һ������ɫ��Ϊdz�Ϻ�ɫ��
��4�������к͵ζ��������������ʵ������Ӷ�����X��ֵ��
��5����c���⣩=
| c(��)?V(�ζ�) |
| V(��) |
���
�⣺��1��KMnO4��Һ����ǿ�����ԣ����Ը�ʴ��Ƥ�ܣ�Ӧװ����ʽ�ζ����У��ʴ�Ϊ����ʽ��
��2������һ�����ʵ���Ũ�ȵ���Һ��Ҫ����ƿ���ձ������������ζ�������Ҫ�ζ��ܼУ������÷�����ƽ�����Բ���Ҫ�������Тܢޣ��ʴ�Ϊ���ܢޣ�
��3�����ᷴӦ��ϣ��������һ��KMnO4��Һ����Һ��Ϊdz�Ϻ�ɫ����ɫ30s�ڲ���ȥ��˵���ζ����յ㣬
�ʴ�Ϊ���������һ��KMnO4��Һ����Һ��Ϊdz�Ϻ�ɫ����30s��dz�Ϻ�ɫ����ȥ��
��4�������εζ����̫������ǰ���εζ�����������ø��������Һ�����Ϊ��10.00mL��1.260g���ᾧ�����������Һ��������������ʵ���Ϊ��4��
��0.1000mol/L��0.010L=0.010mol��X=
��(
-90)=2���ʴ�Ϊ��2��
��5���ٵζ���ʼʱ���ӵζ��̶ܿȣ��ζ�����ʱ���ӵζ��̶ܿȣ������Һ�����С���������ʵ�����С��������С����ˮƫ�࣬Xֵƫ�ߣ��ʴ�Ϊ��ƫ�ߣ�
�ڸ�����ر�ҺŨ��ƫ�ͣ����ñ�Һ���ƫ������IJ������ʵ���ƫ�࣬����ƫ��ˮ������ƫС��XƫС���ʴ�Ϊ��ƫ�ͣ�
��2������һ�����ʵ���Ũ�ȵ���Һ��Ҫ����ƿ���ձ������������ζ�������Ҫ�ζ��ܼУ������÷�����ƽ�����Բ���Ҫ�������Тܢޣ��ʴ�Ϊ���ܢޣ�
��3�����ᷴӦ��ϣ��������һ��KMnO4��Һ����Һ��Ϊdz�Ϻ�ɫ����ɫ30s�ڲ���ȥ��˵���ζ����յ㣬
�ʴ�Ϊ���������һ��KMnO4��Һ����Һ��Ϊdz�Ϻ�ɫ����30s��dz�Ϻ�ɫ����ȥ��
��4�������εζ����̫������ǰ���εζ�����������ø��������Һ�����Ϊ��10.00mL��1.260g���ᾧ�����������Һ��������������ʵ���Ϊ��4��
| 5 |
| 2 |
| 1 |
| 18 |
| 1.260g |
| 0.0100mol |
��5���ٵζ���ʼʱ���ӵζ��̶ܿȣ��ζ�����ʱ���ӵζ��̶ܿȣ������Һ�����С���������ʵ�����С��������С����ˮƫ�࣬Xֵƫ�ߣ��ʴ�Ϊ��ƫ�ߣ�
�ڸ�����ر�ҺŨ��ƫ�ͣ����ñ�Һ���ƫ������IJ������ʵ���ƫ�࣬����ƫ��ˮ������ƫС��XƫС���ʴ�Ϊ��ƫ�ͣ�
���������⿼����������ԭ�ζ���ע��ζ��ܵ�ʹ�ú͵ζ����㣬��Ŀ�ѶȽϴ�

��ϰ��ϵ�д�
 Сѧ�̲���ȫ���ϵ�д�
Сѧ�̲���ȫ���ϵ�д�
�����Ŀ
�����������ڵ���ʵ��ǣ�������
| A������ | B������NaOH |
| C��NaCl��Һ | D��ͭ |
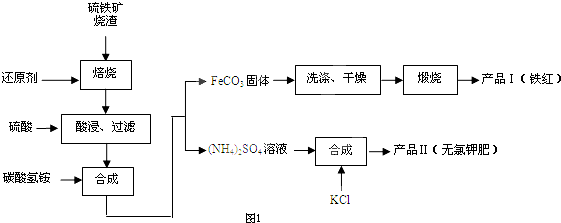
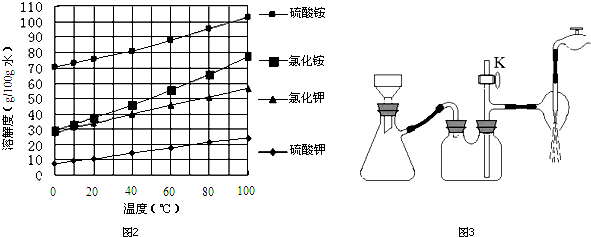
�������зḻ��ʳƷ���������Դ��ҩ���ˮ����Դ����ͼΪ��ˮ���õIJ��ֹ��̣������й�˵����ȷ���ǣ�������


| A���ó����ʯ��ˮ�ɼ���NaHCO3��Na2CO3 |
| B����ȡNaHCO3�ķ�Ӧ���������ܽ��С��NaCl |
| C���ڵڢۡ��ܡ��ݲ����У���Ԫ�ؾ������� |
| D����ҵ��ͨ����ⱥ��MgCl2��Һ��ȡ����þ |
��ij�ܱ������м���0.3mol A��0.1mol C��һ������B�������壬һ�������·�����Ӧ��������Ũ����ʱ��仯��ͼ1��ʾ��ͼ2Ϊt2ʱ�̺�ı�������������ƽ����ϵ��������ʱ��仯����������ĸ��ζ����ı�һ��������������������ͬ����֪t3��t4��Ϊʹ�ô���������˵��������ǣ�������

| A����t1=15s��������C�� t0��t1ʱ��ε�ƽ����Ӧ����Ϊ0.004 mol?L-1?s-1 |
| B��t4��t5�θı������Ϊ��Сѹǿ��t5��t6�θı������������ѧ��Ӧ���¶� |
| C��B����ʼ���ʵ���Ϊ0.02 mol |
| D���û�ѧ��Ӧ�ı���ʽΪ��3A��g��?B��g��+2C��g�� |
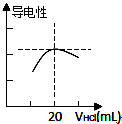
����ʱ����V1mL��c1mol?L-1�İ�ˮ�μӵ�V2mL��c2mol?L-1�������У���������һ����ȷ���ǣ�������
| A����c1=c2�����Һ��c��NH4+��=c��Cl-������V1��V2 |
| B���������Һ��pH=7����c1V1��c2V2 |
| C����V1��V2��c1=c2������Һ��pH��7 |
| D���������Һ��pH��7������Һ��c��NH4+����c��OH-����c��Cl-����c��H+�� |
��ʯī�缫�������M��NO3��x��ˮ��Һ��������������a gʱ����������ͬʱ����b L��������״�������Ӷ���֪M�����ԭ������Ϊ��������
A��
| ||
B��
| ||
C��
| ||
D��
|
 ijѧϰС�������кͷ�Ӧԭ���ⶨij��ˮ�����ʵ���Ũ�ȣ��Բ�����Һ�������жϵζ��յ㣮ʵ�鲽�����£�
ijѧϰС�������кͷ�Ӧԭ���ⶨij��ˮ�����ʵ���Ũ�ȣ��Բ�����Һ�������жϵζ��յ㣮ʵ�鲽�����£�