��Ŀ����
15��Ϊ��̽��������̬�����SO2��NO2��CO2�������ʣ�ijͬѧ�����һ��ʵ�飺ʵ��һ��̽������������ˮ�е��ܽ��ԣ�����֧��ͬ���Թ��ռ����������壬������ʢ��ˮ���ձ��У�һ��ʱ��۲쵽��������ͼA��B��C��ʾ��

��1������ͬ�����£�����������ˮ���ܽ��������NO2����A����
д��A�ձ��з�����Ӧ�Ļ�ѧ����ʽ��3NO2+H2O�T2HNO3+NO��
ʵ���������ֻ����ƿ�ռ��������������������壬Ȼ���䵹����ˮ���У��ֱ���ͨ������O2��Cl2����ͼD��E��F��ʾ��һ��ʱ���D��Eװ�õļ���ƿ�г�����Һ��Fװ�õļ���ƿ�л�������ʣ�࣮

��2��ʵ�����װ��D�ļ���ƿ���ճ�����Һ������ƿ��Һ�岻��ɢ����
��д��װ��D���ܷ�Ӧ�Ļ�ѧ����ʽ��4NO2+O2+2H2O�T4HNO3��
�ڼ����ʵ�������£�����Ħ�����Ϊa L•mol-1����װ��D�ļ���ƿ��������Һ���ʵ����ʵ���Ũ��Ϊ$\frac{1}{a}$mol/L��
��3��ʵ��ǰ��Fװ�õ�ˮ����μӼ�����ɫʯ����Һ���۲쵽����������ɫʯ����Һ��죬ͨ���������ܹ۲쵽��ʵ�������Ǻ�ɫ��dz���д����Ӧ�Ļ�ѧ����ʽ��2SO2+O2+2H2O=2H2SO4��
��4����Һ��������ƿ����Eװ�õ�ˮ����μ����ᱵ��Һ��д���йط�Ӧ�����ӷ���ʽSO2+Cl2+2H2O=4H++SO42-+Cl-��SO42-+Ba2+=BaSO4����
���� ��1��������������̼���ܽ�Ȳ�����������ˮ����������ԭ��Ӧ�����ܽ�����������������ˮ�õ�����Һ��Ϊ����Һ��
��2����D�ж���������ˮ��������Ӧ�������
��ˮ���������ƿ�����c=$\frac{n}{V}$���㣻
��3��Fװ���ж���������ˮ��Ӧ���������ᣬ��Һ�����ԣ���ͨ������������2SO2+O2+2H2O=H2SO4��������ǿ��
��4��Eװ�÷���SO2+Cl2+2H2O=H2SO4+2HCl���ٵμ����ᱵ��Һ���������ᱵ������
��� �⣺��1����ͼA��B��C��ʵ�����������Թ���Һ������Խ�ߣ�������ͬ������������ˮ���ܽ��Խ����A��ʣ���������������ж��Ƕ�����������ˮ������Ӧ����NO��3NO2+H2O�T2HNO3+NO��
�ʴ�Ϊ��NO2����A����3NO2+H2O�T2HNO3+NO��
��2����װ��D�з����ķ�Ӧ�У�3NO2+H2O�T2HNO3+NO��2NO+O2�T2NO2����ӵ��ܷ�Ӧ����ʽ��4NO2+O2+2H2O�T4HNO3��
�ʴ�Ϊ��4NO2+O2+2H2O�T4HNO3��
��ˮ���������ƿ���輯��ƿ���ΪVL�������Һ�����ΪVL����4NO2+O2+2H2O�T4HNO3֪��n��NO2��=n��HNO3������������Һ���ʵ����ʵ���Ũ��Ϊc=$\frac{n}{V}$=$\frac{\frac{VL}{amol/L}}{VL}$=$\frac{1}{a}$mol/L��
�ʴ�Ϊ��$\frac{1}{a}$mol/L��
��3��Fװ���ж���������ˮ��Ӧ���������ᣬ��Һ�����ԣ��μӼ�����ɫʯ����Һ���۲쵽����������ɫ��Һ��죬��ͨ������������2SO2+O2+2H2O=2H2SO4��������ǿ����ɫ��dz���
�ʴ�Ϊ����ɫʯ����Һ��죻��ɫ��dz���2SO2+O2+2H2O=2H2SO4��
��4��Eװ�÷���SO2+Cl2+2H2O=H2SO4+2HCl�����ӷ�ӦΪ��SO2+Cl2+2H2O=4H++SO42-+Cl-���ٵμ����ᱵ��Һ���۲쵽�������ᱵ��ɫ���������ӷ�ӦΪ��SO42-+Ba2+=BaSO4����
�ʴ�Ϊ��SO2+Cl2+2H2O=4H++SO42-+Cl-��SO42-+Ba2+=BaSO4����
���� ���⿼������ʵ�鷽������ƣ�Ϊ��Ƶ���㣬��Ŀ�Ѷ��еȣ��������ʵ����ʼ�ʵ������з����ķ�ӦΪ���Ĺؼ������ط���������ʵ���������ۺϿ��飮

 ����ν����Ž̲��㽭���̴�ѧ������ϵ�д�
����ν����Ž̲��㽭���̴�ѧ������ϵ�д� �����Ļ������������������ϵ�д�
�����Ļ������������������ϵ�д���������
| ��������� | ���� | |
| A | 50mL 1mol/L H2SO4 | ��Ӧ������c��Na+��=c��SO42-�� |
| B | 0.5mol CaO | ����Ӧ��ȫ����Һ�������Ϊ100mL������Һ��pHԼΪ14 |
| C | 50mL H2O | c��Na+��=2[c��CO32-��+c��HCO3-��] |
| D | 0.1mol NaHSO4 | ��Ӧ��ȫ����ҺpH��С��c ��Na+������ |
| A�� | A | B�� | B | C�� | C | D�� | D |
| A�� | Cl2���к�ǿ�������ԣ��ڻ�ѧ��Ӧ��ֻ���������� | |
| B�� | Cl-��ClΪ��ͬ�ĺ��أ��в�ͬ�Ļ�ѧ���� | |
| C�� | ʵ�����Ʊ�Cl2�������ű���ʳ��ˮ�������ռ� | |
| D�� | ��ⱥ��ʳ��ˮ������ʱ�����Դ����������ʯī���Ϸ���������ɫ���� |
| A�� | �轺��ף���ˮ����ǿ������õ�ʳƷ����� | |
| B�� | ��Ļ�ѧ���ʲ����ã������²����κ�������Ӧ | |
| C�� | ��ĵ������ܽ��ڵ���;�Ե��֮�䣬�����õİ뵼����� | |
| D�� | Na2SiO3��Һ�׳�ˮ������������ľ�ķ���� |
| A�� | 127I��131I��Ϊͬ�������� | B�� | 137Cs�ĺ˵����Ϊ137 | ||
| C�� | ��235����������143 | D�� | ���ˮ�ķ�Ӧ������ˮ�ķ�Ӧ���� |
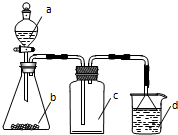 ʵ������ijЩ�������ȡ���ռ���β������װ����ͼ��ʾ��ʡ�Լгֺ;���װ�ã����ô�װ�úͱ����ṩ��������������ʵ����ǣ�������
ʵ������ijЩ�������ȡ���ռ���β������װ����ͼ��ʾ��ʡ�Լгֺ;���װ�ã����ô�װ�úͱ����ṩ��������������ʵ����ǣ�������| ѡ�� | a������ | b������ | c�е����� | d������ |
| A | Ũ��ˮ | CaO | NH3 | H2O |
| B | ϡ���� | Cu | NO | H2O |
| C | ���� | Na2CO3 | CO2 | NaOH��Һ |
| D | Ũ���� | MnO2 | Cl2 | NaOH��Һ |
| A�� | A | B�� | B | C�� | C | D�� | D |
| A�� | NH4+��NO3-��Al3+��K+ | B�� | Na+��NO3-��S2-��K+ | ||
| C�� | MnO4-��SO32-��Na+��K+ | D�� | HCO3-��SO42-��Na+��K+ |
 ��XΪ±��ԭ�ӣ�
��XΪ±��ԭ�ӣ� �����ʣ���������һ�����ϣ�
�����ʣ���������һ�����ϣ�
 ��
�� ��
�� ��
��