��Ŀ����
1���л���A������ʳƷ��ҵ����֪9.0g A������O2�г��ȼ�գ������ɵĻ����������ͨ��������Ũ����ͼ�ʯ�ң��ֱ�����5.4g��13.2g��������ʣ������ΪO2����1��A���ӵ�����ͼ��ͼ��ʾ����ͼ�п�֪����Է���������90����A�ķ���ʽ��C3H6O3��

��2��A����NaHCO3��Һ������Ӧ��Aһ�����еĹ��������Ȼ���
��3��A���ӵĺ˴Ź���������4�����շ壬�����֮����1��1��1��3����A�Ľṹ��ʽ��CH3CH��OH��COOH��
��4����д����������A��ͬ��ͬ���칹��Ľṹ��ʽHOCH2CH2COOH��
���� ��1����ͼA���ӵ�����ͼ����֪A����Է�������Ϊ90�������л���A��������̼��ˮ�����ʵ���������ԭ���غ�ȷ��C��Hԭ����Ŀ�������Է�������ȷ����ԭ����Ŀ������ȷ���л������ʽ��
��2��A����NaHCO3��Һ������Ӧ��Aһ�������Ȼ���
��3��A���ӵĺ˴Ź���������4�����շ壬˵������4��Hԭ�ӣ������֮����1��1��1��3����4��Hԭ����Ŀ��Ϊ1��1��1��4������л������ʽ�뺬�еĹ�����ȷ����ṹ��ʽ��
��4�����A�Ľṹ��ʽ��д��������ͬ��A���ܵ�ͬ���칹�壮
��� �⣺��1����ͼA���ӵ�����ͼ����֪A����Է�������Ϊ90��9gA�����ʵ���Ϊ$\frac{9g}{90g/mol}$=0.1mol��ȼ�����ɶ�����̼Ϊ$\frac{13.2g}{44g/mol}$=0.3mol������ˮΪ$\frac{5.4g}{18g/mol}$=0.3mol�����л��������N��C��=$\frac{0.3mol}{0.1mol}$=3��N��H��=$\frac{0.3mol��2}{0.1mol}$=6���������N��O��=$\frac{90-12��3-6}{16}$=3�����л���A�ķ���ʽΪ��C3H6O3��
�ʴ�Ϊ��90��C3H6O3��
��2��A����NaHCO3��Һ������Ӧ��Aһ�������Ȼ����ʴ�Ϊ���Ȼ���
��3���л���A�ķ���ʽΪ��C3H6O3�������Ȼ���A���ӵĺ˴Ź���������4�����շ壬˵������4��Hԭ�ӣ������֮����1��1��1��3����4��Hԭ����Ŀ��Ϊ1��1��1��4����A�Ľṹ��ʽΪ��CH3CH��OH��COOH��
�ʴ�Ϊ��CH3CH��OH��COOH��
��4����������A��ͬ��ͬ���칹��Ľṹ��ʽ��HOCH2CH2COOH��
�ʴ�Ϊ��HOCH2CH2COOH��
���� ���⿼���л������ʽ��ṹʽ��ȷ������������ͬ���칹����д������ȼ�շ�����ԭ���غ�ȷ���л������ʽ��

| A�� | �ӳɷ�Ӧ | B�� | �ۺϷ�Ӧ | C�� | ˮ�ⷴӦ | D�� | ������Ӧ |
| A�� | ���� | B�� | ����� | C�� | ̼������Һ | D�� | ����������Һ |
| A�� | ̼��������ᷴӦ��CO32-+2H+�TCO2��+H2O | |
| B�� | ����ϡ���ᷴӦ��Fe+2H+�TFe2++H2�� | |
| C�� | ������ͨ�������У�NH3+HCl�TNH4++Cl- | |
| D�� | ����������Һ�еμ�ϡ���OH-+H+�TH2O |
| A�� | �������ΪZn��Al��w��������0.1mol | |
| B�� | �������ΪZn��Fe��w���ܴ���0.1mol | |
| C�� | �������ΪFe��Mg��������ϡ����������������������9.8% | |
| D�� | �������ΪCu��Fe��w��0.05mol����������Fe��Cu��������Ϊ1��1 |
| A�� | �õ�ص�������ӦΪ��O2+2H2O+4e-�T4OH- | |
| B�� | �õ�صĸ�����ӦΪ��C8H18+25O2--50e-�T8CO2��+9H2O | |
| C�� | ����ʵ������Ǵ��ݵ��� | |
| D�� | ������������������ƶ� |

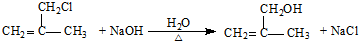 ����Ļ�ѧ����ʽ��
����Ļ�ѧ����ʽ��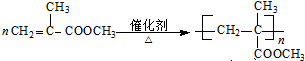 ��
�� ����Ԫ�����ڱ��е�������Ԫ�ص����֪ʶ���ش��������⣺
����Ԫ�����ڱ��е�������Ԫ�ص����֪ʶ���ش��������⣺ ��CNO-������ԭ�ӵ��ӻ���ʽΪsp��
��CNO-������ԭ�ӵ��ӻ���ʽΪsp��