��Ŀ����
4��A��B��C��D��E���ֿ����Ի�����ֱ���������Fe3+��Ba2+��Al3+��Na+��Ag+��������NO3-��OH-��SO42-��Cl-��CO32-�еĸ�һ����ɣ����Ӳ��ظ�������������ʵ�飺��A��E ����Һ�Լ��ԣ�0.1mol/L A��Һ��pHС��13��
����B ����Һ����μ��백ˮ�а�ɫ�������ɣ������Ӱ�ˮ��������������ʧ��
����C ����Һ�м������ۣ���Һ���������ӣ�
����D ����Һ�м��������Ba��OH��2��Һ��û�г�����
��1������������ʵ�ƶ�A-E�Ļ�ѧʽ��
ANa2CO3��BAgNO3��CFe2��SO4��3��EBa��OH��2
��2��д����A��Һ�ʼ��Ժ͢۵����ӷ���ʽ��
��CO32-+H2O?HCO3-+OH-��
��2Fe3++Fe�T3Fe2+��
���� ��A��E��Һ�Լ��ԣ�Ϊ���ǿ�������Σ��������ӹ����֪����ΪBa��OH��2��Na2CO3������0.1mol/L��A��Һ��pHС��13������A��Na2CO3��E��Ba��OH��2��
����B��Һ�����백ˮ�а�ɫ�������ɣ��������백ˮ��������������ʧ����B��Һ�к���Ag+����������ΪNO3-������BΪAgNO3��Һ��
����C��Һ�м������ۣ���Һ���������ӣ�˵��C��Һ����Fe3+��������Һ�����Ӳ��ظ����֣�����D��Һ�к���Al3+��
����D��Һ�м������Ba��OH��2��Һ��û�г�����˵��D��Һ������SO42-������������ΪCl-��SO42-��C��Һ�У�����C��Fe2��SO4��3��D��AlCl3���Դ˽����⣮
��� �⣺��A��E��Һ�Լ��ԣ�Ϊ���ǿ�������Σ��������ӹ����֪����ΪBa��OH��2��Na2CO3������0.1mol/L��A��Һ��pHС��13������A��Na2CO3��E��Ba��OH��2��
����B��Һ�����백ˮ�а�ɫ�������ɣ��������백ˮ��������������ʧ����B��Һ�к���Ag+����������ΪNO3-������BΪAgNO3��Һ��
����C��Һ�м������ۣ���Һ���������ӣ�˵��C��Һ����Fe3+��������Һ�����Ӳ��ظ����֣�����D��Һ�к���Al3+��
����D��Һ�м������Ba��OH��2��Һ��û�г�����˵��D��Һ������SO42-������������ΪCl-��SO42-��C��Һ�У�����C��Fe2��SO4��3��D��AlCl3��
��1�������Ϸ�����֪��AΪNa2CO3��BΪAgNO3��CΪFe2��SO4��3��EΪBa��OH��2���ʴ�Ϊ��Na2CO3��AgNO3��Fe2��SO4��3��Ba��OH��2��
��2��AΪNa2CO3��Ϊǿ�������Σ�ˮ��ʼ��ԣ�ˮ�����ӷ���ʽΪCO32-+H2O?HCO3-+OH-��
C��Fe2��SO4��3��Һ���������������ۺ�Ӧ�����ӷ���ʽΪ2Fe3++Fe=3Fe2+��
�ʴ�Ϊ��CO32-+H2O?HCO3-+OH-�� 2Fe3++Fe�T3 Fe2+��
���� ���������ӹ�������ӷ�ӦΪ���忼��������ƶϣ�����ѧ���ķ��������Ŀ��飬�Դ˶������ƶϣ��Ǹ߿����ȵ���ѵ㣮�ڽ������ʱ�����Ƚ������������������Ƴ���Ȼ�����Ƴ��������Ƶ�ʣ������ʣ���������֤���ɣ�

| A�� | 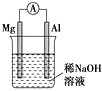 ͼ��Ƭ�����ĵ缫��Ӧʽ�ǣ�Al+4OH-3e-�TAlO2-+2H2O | |
| B�� |  ͼ�������ⸯʴ�����ӷ�Ӧ����ʽΪ��Fe+2H+�TFe2++H2�� | |
| C�� | 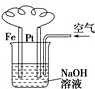 ͼ��Һ�з����˱仯��4Fe��OH��2+O2+2H2O�T4Fe��OH��3 | |
| D�� | 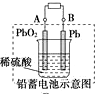 ͼ���ʱ��������Ӧ�ǣ�PbSO4+2H2O-2e-�T=PbO2+SO42-+4H+ |
| A�� | �ƹ�ʹ�ú���ϴ�Ӽ� | B�� | �ù�ҵ��ˮֱ�ӹ��ũ�� | ||
| C�� | ��O3���Cl2������ˮ������ | D�� | ��Hg2+�ķ�ˮֱ���ŷ� |
| A�� | SO2��ˮ��Һ���Ե���˵����SO2����� | |
| B�� | ����NaOH��Һʱ����ʹ�ò����Թܣ���Ϊ�����е�SiO2����NaOH��Ӧ | |
| C�� | ij��ɫ��Һ�м�������������Һ�����ȣ�������������ʹʪ���ɫʯ����ֽ����������Һһ����NH4+ | |
| D�� | ij��Һ�м��������ܲ���ʹ����ʯ��ˮ����ǵ����壬�����Һ��һ������CO32-��HCO3- |
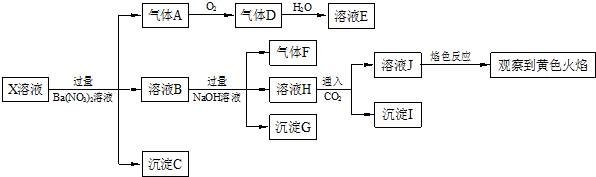
���н�����ȷ���ǣ�������
| A�� | X�п϶�����Na+��Fe2+��A13+��NH4+��SO42- | |
| B�� | ����F����������ֱ����������D | |
| C�� | ����Cһ����BaSO4������Gһ����Fe��OH��3������Iһ����Al��OH ��3 | |
| D�� | X�в���ȷ���������� A13+��Na+��K+��C1- |
| A�� | ̼����������Һ��Ӧ��CaCO3+2H +�TCa2++CO2��+H2O | |
| B�� | ����ʯ��ˮ�����ᷴӦ��OH-+H+�TH2O | |
| C�� | ��Na2O2����ˮ��Na2O2+H2O�T2Na++2OH-+O2�� | |
| D�� | ƫ��������Һͨ�����CO2��AlO2-+4CO2+2 H2O�TAl3++4 HCO3- |
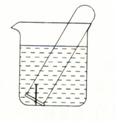 ͼ��ʾˮ���е��Թ�����һö��������������̿�������������۲죺
ͼ��ʾˮ���е��Թ�����һö��������������̿�������������۲죺