��Ŀ����
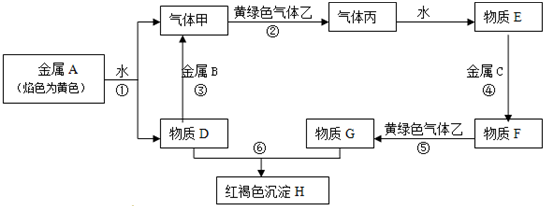
ij������������Fe��Al��Cu���ֽ�����ɣ�Ϊ�ⶨ�������ĺ�����ȡ5.00g ��Ʒ��������ʵ�飺
��ش𣺣�1������A��B��ʵ����������� ��
��2����A��ͨ��Cl2��������Ӧ�����ӷ���ʽ�ǣ�
��3������˵����ȷ���� ������ĸ����
a��ʵ���п����������������
b����A�еμ�KSCN��Һ����Һ��ɺ�ɫ
c��D����ʧˮ���ɵ����ʳ�������ɫ����
��4������������������������Ϊ ��

��ش𣺣�1������A��B��ʵ�����������
��2����A��ͨ��Cl2��������Ӧ�����ӷ���ʽ�ǣ�
��3������˵����ȷ����
a��ʵ���п����������������
b����A�еμ�KSCN��Һ����Һ��ɺ�ɫ
c��D����ʧˮ���ɵ����ʳ�������ɫ����
��4������������������������Ϊ
���㣺̽�����ʵ���ɻ�������ʵĺ���
ר�⣺
�����������̿�֪������������������������������ܽ⣬���˵õ�����BΪCu������Һ�м����������������ӱ�����Ϊ�����ӣ���Һ����Ҫ���������ӡ������ӣ�������������������Һ������ת��Ϊ���������������������γ�ƫ��������ӣ����˵õ�����DΪFe��OH��3��
��1�����ݷ����Һ�����IJ���������
��2���������������ԣ��ɰ��������������������ӣ��ݴ���д��Ӧ�����ӷ���ʽ��
��3��a������Ҳ�ܺ�ͭ��Ӧ��
b�����������Ӧ�����Ȼ��������������ò����Ȼ�����
c�����������ֽ�Ϊ��������Ϊ����ɫ���壬��������ɫȾ�ϣ�
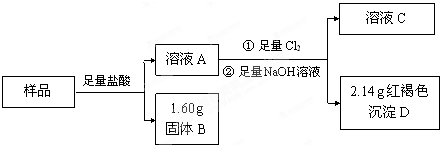
��4������ͭ�������Ӧ����֪BΪͭ������Ϊ1.60g��DΪ���������������ݴ˿ɼ���������������ɴ˿ɼ������������������������
��1�����ݷ����Һ�����IJ���������
��2���������������ԣ��ɰ��������������������ӣ��ݴ���д��Ӧ�����ӷ���ʽ��
��3��a������Ҳ�ܺ�ͭ��Ӧ��
b�����������Ӧ�����Ȼ��������������ò����Ȼ�����
c�����������ֽ�Ϊ��������Ϊ����ɫ���壬��������ɫȾ�ϣ�
��4������ͭ�������Ӧ����֪BΪͭ������Ϊ1.60g��DΪ���������������ݴ˿ɼ���������������ɴ˿ɼ������������������������
���
�⣺��1��BΪ���壬AΪҺ�壬�����Һ�����IJ���Ϊ���ˣ��ʴ�Ϊ�����ˣ�
��2���������������ԣ��ɰ��������������������ӣ���Ӧ�����ӷ���ʽΪ��2Fe2++Cl2�T5Fe3++2Cl-���ʴ�Ϊ��2Fe2++Cl2�T5Fe3++2Cl-��
��3��a������Ҳ�ܺ�ͭ��Ӧ�����ⶨͭ��������������a����
b�����������Ӧ�����Ȼ��������������Ȼ����������軯�ػ��������b����
c�����������ֽ�Ϊ��������Ϊ����ɫ���壬��������ɫȾ�ϣ���c��ȷ��
�ʴ�Ϊ��c��
��4������ͭ�������Ӧ����֪BΪͭ������Ϊ1.60g��DΪ������������������Ϊ��2.14g�����ʵ���Ϊ��
-=0.02mol��������������0.02mol��56g/mol=1.12g������������5.00g-1.60g-1.12g=2.28g����������������
��100%=45.6%���ʴ�Ϊ��45.6%��
��2���������������ԣ��ɰ��������������������ӣ���Ӧ�����ӷ���ʽΪ��2Fe2++Cl2�T5Fe3++2Cl-���ʴ�Ϊ��2Fe2++Cl2�T5Fe3++2Cl-��
��3��a������Ҳ�ܺ�ͭ��Ӧ�����ⶨͭ��������������a����
b�����������Ӧ�����Ȼ��������������Ȼ����������軯�ػ��������b����
c�����������ֽ�Ϊ��������Ϊ����ɫ���壬��������ɫȾ�ϣ���c��ȷ��
�ʴ�Ϊ��c��
��4������ͭ�������Ӧ����֪BΪͭ������Ϊ1.60g��DΪ������������������Ϊ��2.14g�����ʵ���Ϊ��
| 2.14g |
| 107g/mol |
| 2.28g |
| 5.00g |
���������⿼���˻�������������IJⶨ���е��Ѷȣ�����Ԫ�ػ��������ʼ��ɽ����ע�����֪ʶ�Ļ��ۣ�

��ϰ��ϵ�д�
 ������������Ӧ����ϵ�д�
������������Ӧ����ϵ�д� ͬ����չ�Ķ�ϵ�д�
ͬ����չ�Ķ�ϵ�д�
�����Ŀ
��NA��ʾ�����ӵ�����������˵����ȷ���ǣ�������
| A�����³�ѹ�£�1mol��ϩ���õ��Ӷ���Ϊ4NA |
| B��1mol��ȩ����������Cu��OH��2����Һ��Ӧ��ת�Ƶ�����ĿΪNA |
| C����״���£�1L������ȼ�պ�������̬����ķ�����Ϊ5/22.4 NA |
| D��0.1mol��ϩ���Ҵ��Ļ������ȫȼ�������ĵ���ԭ����һ��Ϊ0.6 NA |
������������·�Ӧ��C4H10O���ף�
C4H8���ң�
C4H8Br2�������õ����������ܵ��ǣ�������
| ŨH2SO4 |
| �� |
| Br2 |
| CCl4 |
| A��CH3CH2CHBrCH2Br |
| B��CH3CH��CH2Br��2 |
| C��CH3CHBrCHBrCH3 |
| D����CH3��2CBrCH2Br |
�о�������̼���ʵ���ʵ��װ����ͼ������70%������Һ���������ƾ��巴Ӧ��ȡSO2���壬ʵ����������ԣ��Ҳ�����Ⱦ����������˵���д�����ǣ�������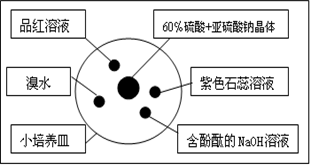

| A����ˮ��ɫ��ȥ��ԭ����SO2�������Ư���� |
| B������̪��NaOH��Һ��ɫ��dz��ԭ����SO2�������Ư���� |
| C��Ʒ����Һ��ɫ��ԭ����SO2�������Ư���� |
| D����ɫʯ����Һ����ɫ��ԭ����SO2����������Ժ�Ư���� |
���������г��õ�CO��H2����ͨ������ˮ������Ӧ�õ������м���ɷ�������������Ӧ��
��1��CH4��g��+
O2��g��=2H2��g��+CO��g������H=-36kJ/mol
��2��CH4��g��+H2O��g��=CO��g��+3H2��g������H=+216kJ/mol
���л�ѧ��Ӧ����ʽ�У���Ӧ��Ϊ����ǣ�������
��1��CH4��g��+
| 1 |
| 2 |
��2��CH4��g��+H2O��g��=CO��g��+3H2��g������H=+216kJ/mol
���л�ѧ��Ӧ����ʽ�У���Ӧ��Ϊ����ǣ�������
| A��7CH4��g��+3O2��g��+H2O��g��=7CO��g��+15H2��g�� |
| B��5CH4��g��+2O2��g��+H2O��g��=5CO��g��+11H2��g�� |
| C��4CH4��g��+O2��g��+2H2O��g��=4CO��g��+10H2��g�� |
| D��3CH4��g��+O2��g��+H2O��g��=3CO��g��+7H2��g�� |
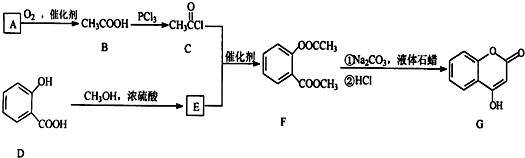
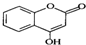 ��һ��ҽҩ�м��壬�������Ʊ�����Ѫҩ����ͨ������·�ߺϳ�
��һ��ҽҩ�м��壬�������Ʊ�����Ѫҩ����ͨ������·�ߺϳ�