��Ŀ����
20��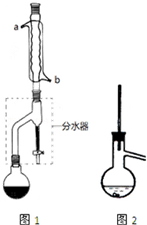 �л���ķ�Ӧ�������渱��Ӧ�����������Ҫ�����ᴿ����һ��ˮ���㾫�ĺϳɲ������£�
�л���ķ�Ӧ�������渱��Ӧ�����������Ҫ�����ᴿ����һ��ˮ���㾫�ĺϳɲ������£���ϳɣ�
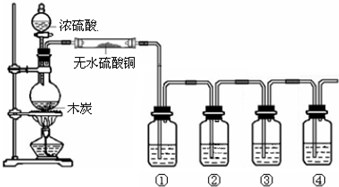
�ڸ����Բ����ƿ�м�11.5mL��9.3g��0.125mol����������7.2mL��7.5g��0.125mol�������ᣨ���ᣩ��3��4��ŨH2SO4��ҡ�ȺӼ�����ʯ���ٰ�ͼ1��ʾװ�ð�װ�ã��ڷ�ˮ����Ԥ�ȼ���5.00mLˮ����ˮ����ڷ�ˮȥ����֧������3��5mm��Ȼ����С����ȣ���Ӧ��Լ40min��
������ᴿ��
��%2����ˮ���е�Һ�治������ʱ����ȴ���ų���ˮ���е�ˮ���ѷ�Ӧ�����Һ���ˮ���е�����ϲ���ת���Һ©���У���10mL10%̼������Һϴ�����������ԣ�pH=7����������ã���ȥˮ�㣮
�ڽ����㵹��С��ƿ�У���������ˮ����þ�������MgSO4•7H2O���壩
�۽������������ֲ�Ʒת��50mL������ƿ�У��Ӽ�����ʯ���г�ѹ�����ռ���Ʒ����Ҫ�Լ�������������������£�
| ������ | ������ | ������ | ���������� | ������ |
| �ܶ�/��g/mL�� | 0.810 | 1.049 | 0.882 | 0.7689 |
| �е�/�� | 117.8 | 118.1 | 126.1 | 143 |
| ��ˮ�е��ܽ��� | ���� | ���� | ���� | ���� |
2CH3CH2CH2CH2OH $?_{��}^{ŨH_{2}SO_{4}}$CH3CH2CH2CH2OCH2CH2CH2CH3+H2O
����������Ϣ�ش��������⣺
��1��д���ϳ������������Ļ�ѧ����ʽCH3CH2CH2CH2OH+CH3COOH$?_{��}^{ŨH_{2}SO_{4}}$CH3COOCH2CH2CH2CH3+H2O��
��2����ͼ1����װ�ÿɿ����ɷ�ˮ����Բ����ƿ����������ɣ�������ˮӦ��b����a��b���ܿ�ͨ�룮
��3���������̼������Һ��������Ҫ�dz�ȥ���ᡢ���ᡢ��������
��4���ڲ�������ں���������ˮ����þ�����Ӧ�ȹ��ˣ���ʵ��������ƣ���Ȼ�������������ֲ�Ʒת��������ƿ�У��������װ����ͼ2��ʾ�����ռ����IJ�Ʒ�п��ܻ������������ʣ�
��5������۵ij�ѹ���������һ�����¶ȣ�����Ϊ��bd�м��ȱȽϺ��ʣ��������ѡ����ѡ��
A��ˮ B�����ͣ��е�290�棩 C��ɳ�� D��ʯ���ͣ��е�200��300�棩
��6����Ӧ���������ų���ˮΪ6.98mL��ˮ���ܶ�Ϊ1g/mL��������������ת����ԼΪ88%��
���� ��1�����������������ŨH2SO4���������·���������Ӧ����������������ˮ��
��2�������ܲ�ȡ����ԭ��ͨ������ˮ����������ȴ��
��3�������������к������ᡢ���������������ʣ�����������������������ˮ�����������������ܣ�������뱥��̼���Ʒ�Ӧ���Դ˽����⣮
��4����������ˮ����þ�����Ӧ���˳�ȥ���������Ӧ�з�������Ӧ�õ������ѣ������������ܣ�����������������ˮ���Ʊ��������к��������ѣ�
��5�������������ķе���126.1�棬ѡ��е��Ըߵ�Һ����ȣ�
��6����������ˮ�����������ݷ���ʽ����μӷ�Ӧ�������IJ�������������ת����=$\frac{�μӷ�Ӧ������}{Ͷ���������}$��100%��
��� �⣺��1�����������������ŨH2SO4���������·���������Ӧ����������������ˮ����ѧ����ʽΪCH3CH2CH2CH2OH+CH3COOH$?_{��}^{ŨH_{2}SO_{4}}$CH3COOCH2CH2CH2CH3+H2O��
�ʴ�Ϊ��CH3CH2CH2CH2OH+CH3COOH$?_{��}^{ŨH_{2}SO_{4}}$CH3COOCH2CH2CH2CH3+H2O��
��2�������ܲ�ȡ����ԭ��ͨ������ˮ��������ˮӦ��b�ܿ�ͨ�룬��a������������������ȴ��
�ʴ�Ϊ��b��
��3�������������к������ᡢ���������������ʣ����������������ڱ���̼������Һ�������������������ܣ��������̼���Ʒ�Ӧ�������գ����ñ���̼������Һ��ȥ���������������ᡢ���������������ʣ��ʴ�Ϊ�����ᡢ���ᡢ��������
��4����������ˮ����þ�����Ӧ���˳�ȥ�������������������������ˮ������ˮϴ��10%̼������Һϴʱ���Ѿ�����ȥ�������������ᶡ�����ܣ�ˮϴ��10%̼������Һϴʱ���ܳ�ȥ����������ʱ���������ӷ��������ᶡ����������������������Ϊ�����ѣ�
�ʴ�Ϊ�����ˣ������ѣ�
��5�������������ķе���126.1�棬ѡ��е��Ըߵ�Һ����ȣ����Կ����ڸ��ͺ�ʯ�����м��ȣ�ˮ�ķе�Ϊ100�棬�����¶�̫�ͣ���ɰ�Ӽ����¶�̫�߲����ƣ�
��ѡ��bd��
��6����Ӧ���������ų���ˮΪ6.98mL��ˮ���ܶ�Ϊ1g•mL-1������Ӧ���ɵ�ˮΪ6.98mL-5.00mL=1.98mL����1.98g����μӷ�Ӧ��������Ϊx��
CH3COOH+CH3CH2CH2CH2OH$?_{��}^{Ũ����}$CH3COOCH2CH2CH2CH3+H2O
74 18
x 1.98g
��x=$\frac{74��1.98g}{18g}$=8.14g��
������������������ת����=$\frac{�μӷ�Ӧ������}{Ͷ���������}$��100%=$\frac{8.14g}{9.3g}$��100%=88%��
�ʴ�Ϊ��88%��
���� ���⿼���л����Ʊ�ʵ�飬�漰ʵ�������ʵ��ԭ�������ʵ����ʵ�Ӧ�á������ᴿ���йط���ʽ�ļ���ȣ���Ŀ�Ѷ��еȣ������ڿ���ѧ����ʵ��̽�����������ݴ���������

| A�� | H2CO3?2H++CO32- | B�� | NaHSO4=Na ++HSO4- | ||
| C�� | NaHCO3=Na++H++CO32- | D�� | NH4Cl=NH4++Cl- |
| ���� | ��Է������� | �ܶ�/��g•mL-1�� | �е�/�� | ˮ���ܽ��� |
| CHCl3 | 119.5 | 1.50 | 61.3 | ���� |
| CCl4 | 154 | 1.59 | 76.7 | ���� |
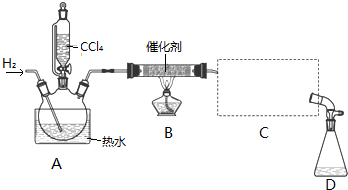
�ټ���װ�������ԣ�
�ڿ�ʼͨ��H2��
�۵�ȼB���ƾ��ƣ�
����A��ˮ���м�����ˮ����ͨC������װ�õ���ˮ��
��������ƿ�е���20mLCCl4��
��Ӧ������ֹͣ���ȣ���D����ƿ���ռ�����Һ��ֱ�������NaHCO3��Һ��ˮϴ�ӣ��ֳ��IJ������������ˮCaCl2���壬���ú���ˣ�
�߶���Һ�����������õ��ȷ�15g����ش�
��1��������ںͲ���۵�˳��ߵ�����ʵ���в����IJ����������Ϊ����ʱ����������������ը�����ɵ��ȷ±�����������
��2��B���з�����Ҫ��Ӧ�Ļ�ѧ����ʽΪCCl4+H2$��_{��}^{����}$CHCl3+HCl��
��3��C����Ӧѡ�õ�������ΪB����ѡ����ĸ������ˮӦ�Ӹ������ܵ�a���a����b�����ڽ��룮
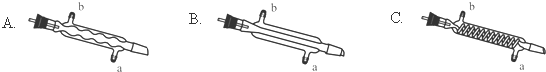
��4��������У���ˮϴ�ӵ�Ŀ��Ϊϴ��NaHCO3��NaCl��
��5����ʵ���У��ȷµIJ���Ϊ61%��
��6���ȷ��ڿ������ܱ�������������HCl������COCl2�����÷�Ӧ�Ļ�ѧ����ʽΪ2CHCl3+O2=2COCl2+2HCl��
��Ӧԭ����
������ϣ�
| ���� | ��Է������� | ��״ | �۵�/�� | �ܽ�� | |
| ˮ | �Ҵ� | ||||
| �ױ� | 92 | ��ɫҺ����ϩ�ӷ� | -95 | ���� | ���� |
| ������ | 122 | ��ɫƬ״����״���� | 122.4��100�������� | 25��0.35g 80��2.7g | ���� |
����ͼ2��װ��Ӧװ�ã�����ˮ���гּ�����װ��δ������������������ƿ�����μ���120mLˮ���Թ���������غ�3.0mL�ױ����ܶ�Ϊ0.866g/mL����
�ڽ�������ƿ�еĻ��Һ����衢���������ڣ�ֱ���ױ���ȫ��Ӧ��
�۳��ȹ��˷�Ӧ��������Һ����ɫ���������������������������Һ����ɫ��ȥ���ٹ��ˣ�����ˮϴ��������ϴ��Һ�ϲ�����Һ�У�
���ñ�ˮ��ȴ��Һ��Ȼ����Ũ�����ữ�����ˣ���������ˮϴ���������õ�������ֲ�Ʒ�����ؽᾧ�õ����Ƶı����ᣮ
�ش��������⣺
��1������ a����Ϊ�����Σ������ܣ�
��2���жϼױ�����ȫ��Ӧ��������������ƿ�л��Һ���ٷֲ㡢����Һ���ٳ������飮
��3��ʵ�鲽����У���������ˮ��������ˮϴ��������Ŀ���Ǽ��ٱ��������ܽ������ʧ��������ֲ�Ʒ���˿����ؽᾧ�������⣬��������������
��4�����Ƶı����ᴿ�Ȳⶨ����ȡ1.220g��Ʒ����ϡ�Ҵ��ܽⲢ���100mL��Һ���ֱ�ȡ25.00mL��Һ����0.1000mo1•L-1NaOH����Һ�ζ������εζ�����NaOH��Һ������ֱ�ΪV1=22.48mL��V2=22.52mL��V3=23.80mL��
��������Һʱ��ϡ�Ҵ�����������ˮ���ܼ���ԭ���dz����±���������ˮ���������Ҵ���
�����÷�̪��ָʾ����ȷ���ζ��յ�������ǵμ����һ���������Ʊ���Һ����Һ����ɫ��Ϊdz��ɫ����30�벻��ɫ��
�۲�Ʒ�Ĵ���Ϊ90%��
| A�� | S2-���ӵĽṹʾ��ͼ��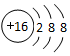 | B�� | �������Ļ�ѧʽ��AlSO4 | ||
| C�� | H2O2��O�Ļ��ϼ�Ϊ-2�� | D�� | ��ԭ�ӵ�ԭ�ӽṹʾ��ͼ��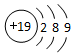 |
 һ���ܱ��������м���һ�����ɻ����ĸ��壨��Ȳ��ƣ��������ֳ������֣�����߳���1molN2���ұ߳���һ������COʱ�����崦����ͼλ�ã������¶Ȳ��䣩������˵����ȷ���ǣ�������
һ���ܱ��������м���һ�����ɻ����ĸ��壨��Ȳ��ƣ��������ֳ������֣�����߳���1molN2���ұ߳���һ������COʱ�����崦����ͼλ�ã������¶Ȳ��䣩������˵����ȷ���ǣ�������| A�� | �ұ�����߷�����֮��Ϊ4��1 | |
| B�� | �Ҳ�CO������Ϊ5.6 g | |
| C�� | �Ҳ������ܶ�����ͬ�����������ܶȵ�14�� | |
| D�� | ���ı��ұ�CO�ij�������ʹ���崦���������м䣬�����¶Ȳ��䣬��Ӧ����0.2molCO |
 ��BA4�Ľṹʽ
��BA4�Ľṹʽ ��
�� ��
��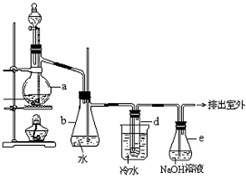 ������±�ᷴӦ���Ʊ�±��������Ҫ������ʵ�����Ʊ��������װ������ͼ��ʾ���Թ�d��װ����������ˮ����֪������ķе�Ϊ38.4oC���ܶ�Ϊ1.43g•ml-1��
������±�ᷴӦ���Ʊ�±��������Ҫ������ʵ�����Ʊ��������װ������ͼ��ʾ���Թ�d��װ����������ˮ����֪������ķе�Ϊ38.4oC���ܶ�Ϊ1.43g•ml-1��