��Ŀ����
����֪Ũ�ȵ�����ζ�ijδ֪Ũ�ȵ�����������Һ������ʵ��ش�
��1��ȷ��ȡ4.1g�ռ���Ʒ������������ҩ�����
��2������Ʒ���250mL����Һ����Ҫ���������ձ���������������
��3��ȡ10.00mL����Һ���� ��ȡ��
��4����0.2010mol?L-1������ζ������ռ���Һ���ζ�ʱ���� ������ ������ע�� ��ֱ���ζ��յ㣮
��5����ѡ�õ�ָʾ��Ϊ ���ζ��յ������ ��
��6�����в�������ʹ�ⶨ�����Σ�����дƫ�ߡ�ƫ�͡���Ӱ�죩
����ʽ�ζ�����װҺǰδ�ñ�������Һ��ϴ2�Ρ�3�Σ�
�ڿ�ʼʵ��ʱ����ʽ�ζ��ܼ��첿�������ݣ��ڵζ������У�������ʧ��
�۵ζ������У���ƿ����Һ�����ʳ���ɫ����ɫ���ٱ�죮
�ܴﵽ�ζ��յ�ʱ��������Һ������͵������ ��
��1��ȷ��ȡ4.1g�ռ���Ʒ������������ҩ�����
��2������Ʒ���250mL����Һ����Ҫ���������ձ���������������
��3��ȡ10.00mL����Һ����
��4����0.2010mol?L-1������ζ������ռ���Һ���ζ�ʱ����
��5����ѡ�õ�ָʾ��Ϊ
��6�����в�������ʹ�ⶨ�����Σ�����дƫ�ߡ�ƫ�͡���Ӱ�죩
����ʽ�ζ�����װҺǰδ�ñ�������Һ��ϴ2�Ρ�3�Σ�
�ڿ�ʼʵ��ʱ����ʽ�ζ��ܼ��첿�������ݣ��ڵζ������У�������ʧ��
�۵ζ������У���ƿ����Һ�����ʳ���ɫ����ɫ���ٱ�죮
�ܴﵽ�ζ��յ�ʱ��������Һ������͵������
���㣺�к͵ζ�
ר�⣺����ƽ������Һ��pHר��
��������1�������ռ���и�ʴ�������׳��⣬ѡ�����ʱʹ�õ�������
��2����������һ�����ʵ���Ũ�ȵ���Һʹ�õ��������
��3�����ݴ���ҺΪ����������Һ��ѡ��ζ������ͣ�
��4���ζ�ʱ������ת��ʽ�ζ��ܵIJ������������ֲ�ͣ��ҡ����ƿ������ע����ƿ����Һ��ɫ�ı仯��
��5����ѡ���̪����ɫΪdz��ɫ��Ϊ��ɫ������ȣ���ɫΪ��ɫ��Ϊ��ɫ����
��6������c�����⣩=
��������������V��������Ӱ�죬�Դ��ж�Ũ�ȵ���
��2����������һ�����ʵ���Ũ�ȵ���Һʹ�õ��������
��3�����ݴ���ҺΪ����������Һ��ѡ��ζ������ͣ�
��4���ζ�ʱ������ת��ʽ�ζ��ܵIJ������������ֲ�ͣ��ҡ����ƿ������ע����ƿ����Һ��ɫ�ı仯��
��5����ѡ���̪����ɫΪdz��ɫ��Ϊ��ɫ������ȣ���ɫΪ��ɫ��Ϊ��ɫ����
��6������c�����⣩=
| V(��)��c(��) |
| V(����) |
���
�⣺��1�������������׳��⣬���к�ǿ�ĸ�ʴ�ԣ�����ʱӦ�÷���С�ձ��г��������Գ���ʹ�õ������У�������ƽ��ҩ�ס��ձ���
�ʴ�Ϊ��������ƽ���ձ���
��2������250ml����Һ����Ҫʹ�õ������У��ձ�������������ͷ�ιܡ�250mL����ƿ��
�ʴ�Ϊ��250mL����ƿ����ͷ�ιܣ�
��3������ҺΪ����������Һ��Ӧ��ʹ�ü�ʽ�ζ��ܣ��ʴ�Ϊ����ʽ�ζ��ܣ�
��4���ζ�ʱ������ת��ʽ�ζ��ܵIJ������������ֲ�ͣ��ҡ����ƿ������ע����ƿ����Һ��ɫ�ı仯��
�ʴ�Ϊ����ʽ�ζ��ܵIJ�����������ͣ��ҡ����ƿ����ƿ����Һ��ɫ�ı仯��
��5����ѡ���̪���۲쵽��ƿ����Һ����ɫ��dz��ɫ��Ϊ��ɫ���ﵽ�ζ��յ㣬��ѡ����ȣ��۲쵽��Һ�ɻ�ɫ��Ϊ��ɫ����ﵽ�ζ��յ㣬
�ʴ�Ϊ����̪������ȣ�����̪Ϊָʾ��ʱdz��ɫ��Ϊ��ɫ�Ұ���Ӳ���ԭ�������Ϊָʾ��ʱ��ɫ��ɫ��ɫ�Ұ���Ӳ���ԭ����
��6������ʽ�ζ�����װҺǰδ�ñ�������Һ��ϴ2�Ρ�3�Σ���ҺŨ��ƫ�ͣ����V������ƫ����c�����⣩=
����������ҺŨ��ƫ��
�ڿ�ʼʵ��ʱ����ʽ�ζ��ܼ��첿�������ݣ��ڵζ������У�������ʧ�����V������ƫ����c�����⣩=
����������ҺŨ��ƫ��
�۵ζ������У���ƿ����Һ�����ʳ���ɫ����ɫ���ٱ�죬��V��������Ӱ�죬����c�����⣩=
����������ҺŨ����Ӱ�죻
�ܴﵽ�ζ��յ�ʱ��������Һ������͵���������V������ƫС������c�����⣩=
����������ҺŨ��ƫ�ͣ�
�ʴ�Ϊ��ƫ��ƫ����Ӱ�죻ƫ�ͣ�
�ʴ�Ϊ��������ƽ���ձ���
��2������250ml����Һ����Ҫʹ�õ������У��ձ�������������ͷ�ιܡ�250mL����ƿ��
�ʴ�Ϊ��250mL����ƿ����ͷ�ιܣ�
��3������ҺΪ����������Һ��Ӧ��ʹ�ü�ʽ�ζ��ܣ��ʴ�Ϊ����ʽ�ζ��ܣ�
��4���ζ�ʱ������ת��ʽ�ζ��ܵIJ������������ֲ�ͣ��ҡ����ƿ������ע����ƿ����Һ��ɫ�ı仯��
�ʴ�Ϊ����ʽ�ζ��ܵIJ�����������ͣ��ҡ����ƿ����ƿ����Һ��ɫ�ı仯��
��5����ѡ���̪���۲쵽��ƿ����Һ����ɫ��dz��ɫ��Ϊ��ɫ���ﵽ�ζ��յ㣬��ѡ����ȣ��۲쵽��Һ�ɻ�ɫ��Ϊ��ɫ����ﵽ�ζ��յ㣬
�ʴ�Ϊ����̪������ȣ�����̪Ϊָʾ��ʱdz��ɫ��Ϊ��ɫ�Ұ���Ӳ���ԭ�������Ϊָʾ��ʱ��ɫ��ɫ��ɫ�Ұ���Ӳ���ԭ����
��6������ʽ�ζ�����װҺǰδ�ñ�������Һ��ϴ2�Ρ�3�Σ���ҺŨ��ƫ�ͣ����V������ƫ����c�����⣩=
| V(��)��c(��) |
| V(����) |
�ڿ�ʼʵ��ʱ����ʽ�ζ��ܼ��첿�������ݣ��ڵζ������У�������ʧ�����V������ƫ����c�����⣩=
| V(��)��c(��) |
| V(����) |
�۵ζ������У���ƿ����Һ�����ʳ���ɫ����ɫ���ٱ�죬��V��������Ӱ�죬����c�����⣩=
| V(��)��c(��) |
| V(����) |
�ܴﵽ�ζ��յ�ʱ��������Һ������͵���������V������ƫС������c�����⣩=
| V(��)��c(��) |
| V(����) |
�ʴ�Ϊ��ƫ��ƫ����Ӱ�죻ƫ�ͣ�
���������⿼������к͵ζ����Ѷ��еȣ�ע������ζ���ԭ���������ע��ָʾ����ѡ��

��ϰ��ϵ�д�
�����Ŀ
���е��뷽��ʽ��ȷ���ǣ�������
| A��H2SO3?2H++SO32- |
| B��HF=H++F- |
| C��NaHS=Na++H++S2- |
| D��H2CO3+H2O?H3O++HCO3- |
����֪Ũ�ȵ�����ζ�δ֪Ũ�ȵ�����������Һʱ������ʵ�����������ⶨ���ƫ�ߵ��ǣ�������ƽ����NaOH����ʱ����NaOH�������̣�����������̣����ƶ�����ʹ֮ƽ�⣮�ڵζ�ǰ�����ݣ��ζ��յ������ݣ���������ˮϴ����ƿ��ʢ�����NaOH��Һ���еζ�����������ˮϴ����ζ��ܺ�ʢ���������еζ����ݵζ��յ��ȡ��ζ�������ʱ�����ӿ̶��ߣ���������
| A���٢� | B���ڢܢ� |
| C���ڢۢܢ� | D���٢ڢۢܢ� |
����ʵ�������װ�ò�����ʵ��Ҫ����ǣ�������
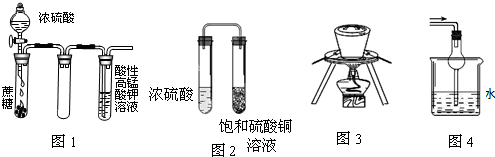

| A��ͼ1����KMnO4��Һ��û�����ݳ��֣�����Һ��ɫ����dz������ȥ |
| B��ͼ2���ú�������ͭ��Һ����������ɫ���� |
| C��ͼ3�ں������ʵ�����������պ��� |
| D��ͼ4����������������ˮ��β�� |
����Fe��OH��2 ���Ʊ��ܹ��ɹ����ǣ�������
| A����FeCl2 ��Һ����μ���NaOH��Һ |
| B����FeSO4��Һ����μ��백ˮ |
| C���Ƚ�ʢ��NaOH��Һ�ij��ιܲ嵽FeSO4Һ���£��ټ���NaOH��Һ���Ƶ�Fe��OH��2�İ�ɫ���� |
| D��ȡ�����Ƶ�FeSO4��Һ���������Թ��У��ڼ���һ��ֲ���ͣ��ܶ�С��ˮ���Ҳ�����ˮ����Ȼ�����Թ�����μ���NaOH��Һ |
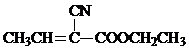 ���Ǻϳ�ij��������ճ�ϼ��ĵ��壬X�ĺϳ�·����ͼ��
���Ǻϳ�ij��������ճ�ϼ��ĵ��壬X�ĺϳ�·����ͼ��