��Ŀ����
1������һ������ˮ��Һ��ֻ���ܺ������������е������֣�K+��NH4+��Cl-��Mg2+��Ba2+��CO32-��SO42-����ȡ����100mL��Һ��������ʵ�飺�ٵ�һ�ݼ���AgNO3��Һ�г����������ڵڶ��ݼ�����NaOH��Һ���Ⱥ��ռ�������0.04mol���۵����ݼ�����BaCl2��Һ�ø������6.27g������������ϴ�ӡ������������Ϊ2.33g���Իش��������⣺��1����ʵ��ڿ�֪��Һ�к��е�����ΪNH4+����100mL��Һ�и����ӵ����ʵ���Ũ��Ϊ0.4mol/L
��2����ʵ��ۿ����ж�����Һ�к��е�����ΪCO32-��SO42-
��3��������ʵ����Ϣ�ж�����˵������ȷ����AC
A��K+һ������ B��100mL��Һ�к�0.01mol CO32-
C��Cl-���ܴ��� D��Ba2+һ�������ڣ�Mg2+���ܴ���
��4����������ʵ�飬����Һ���Ƿ��в��ܿ϶�����ڵ����ӣ����У���Ϊ��һ��ȷ������ڣ�Ӧ�ò����ʵ����ȡ����ԭ��Һ����������Ba��NO3��2��Һ����ȡ�ϲ���Һ����AgNO3��Һ��ϡHNO3������г�������˵������Cl-��
���� �ٵ�һ�ݼ���AgNO3��Һ�г���������˵�����ܺ���Cl-��CO32-��SO42-��
�ڵڶ��ݼ�����NaOH��Һ���Ⱥ��ռ�������0.448L����״̬�£���֤��һ������NH4+�����ɵ�����Ϊ�������ʵ���Ϊ0.04mol���������ɣ�����Һ��һ������Mg2+��Cu2+��
�۵����ݼ�����BaCl2��Һ�ø������6.27g������������ϴ�ӡ������������Ϊ2.33g��֤��ԭ��Һ��һ������CO32-��SO42-������CO32-��SO42-��һ��������Ba2+��
���ɳ���BaSO4���ʵ���=$\frac{2.33g}{233g/mol}$�T0.01mol�����ɵij���BaCO3���ʵ���=$\frac{6.27g-2.33g}{197g/mol}$=0.02mol�����ݵ���غ㣬����K+��Cl-����ȷ�����Դ˽����⣮
��� �⣺�ٵ�һ�ݼ���AgNO3��Һ�г���������˵�����ܺ���Cl-��CO32-��SO42-��
�ڵڶ��ݼ�����NaOH��Һ���Ⱥ��ռ�������0.448L����״̬�£���֤��һ������NH4+�����ɵ�����Ϊ�������ʵ���=0.04mol���������ɣ�����Һ��һ������Mg2+��Cu2+��
�۵����ݼ�����BaCl2��Һ�ø������6.27g������������ϴ�ӡ������������Ϊ2.33g��֤��ԭ��Һ��һ������CO32-��SO42-������CO32-��SO42-��һ��������Ba2+��
���ɳ���BaSO4���ʵ���=$\frac{2.33g}{233g/mol}$�T0.01mol�����ɵij���BaCO3���ʵ���=$\frac{6.27g-2.33g}{197g/mol}$=0.02mol�����ݵ���غ㣬����K+��Cl-����ȷ����
��1����ʵ��ڿ�֪��Һ�к��е�����ΪNH4+����100mL��Һ�и����ӵ����ʵ���Ũ��Ϊ$\frac{0.04mol}{0.1L}$=0.4mol/L���ʴ�Ϊ��NH4+��0.4mol/L��
��2����ʵ��ۿ����ж�����Һ�к��е�����ΪCO32-��SO42-���ʴ�Ϊ��CO32-��SO42-��
��3�������Ϸ�����֪������K+��100mL��Һ�к�0.02mol CO32-��Cl-����ȷ����һ������Mg2+��ֻ��AC��ȷ��
�ʴ�Ϊ��AC��
��4��Cl-����ȷ������ȡ����ԭ��Һ����������Ba��NO3��2��Һ����ȡ�ϲ���Һ����AgNO3��Һ��ϡHNO3������г�������˵������Cl-��
�ʴ�Ϊ��ȡ����ԭ��Һ����������Ba��NO3��2��Һ����ȡ�ϲ���Һ����AgNO3��Һ��ϡHNO3������г�������˵������Cl-��
���� ���⿼��������ƶϣ����ؿ��鳣�����ӵļ��鷽����Ϊ�߿��������ͣ���Ŀ�Ѷ��еȣ������漰�����ݵ���غ��ƶ����ӵĴ��ڣ�Ϊ�״��㣬ע���������ճ������ӵ����ʼ����鷽��������������ѧ���ķ���������������������

 ����������ϵ�д�
����������ϵ�д�| A�� | ��ά�ء������ʡ���֬������Ȼ�л��߷��ӻ����� | |
| B�� | ȼú�м���CaO��ɼ�������ķ���������ŷ� | |
| C�� | ��ά���������ڿ�ˮ��Ϊ�����ǣ���������Ҫ��Ӫ������֮һ | |
| D�� | ������һ��ˮ��������������ˮ��ɱ�������� |
| A�� | 23gNa��ΪNa+ʱʧȥ�ĵ�����ΪNA | B�� | 18gˮ�ĵ�����ΪNA | ||
| C�� | 8gHe�����ķ�����ΪNA | D�� | 16gO2��16gO3������ԭ������ΪNA |
| A�� | �Ȼ��Ƶĵ���ʽΪ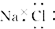 | |
| B�� | þ��ԭ�ӽṹʾ��ͼΪ 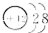 | |
| C�� | �Ȼ�����ӵ��γɹ��̿��õ���ʽ��ʾʽ��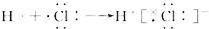 | |
| D�� | ��ˮ�Ļ�ѧʽΪ${\;}_{1}^{2}$H2O����D2O�� |
| A�� | Fe | B�� | Co | C�� | Mn | D�� | Ni |
| A�� | BaCl2 Na2CO3 AgNO3 ���� | B�� | BaCl2 Na2CO3 ���� AgNO3 | ||
| C�� | Na2CO3 ���� AgNO3 BaCl2 | D�� | AgNO3 ���� BaCl2 Na2CO3 |
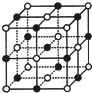 ��֪NiXO���徧���ṹΪNaCl�ͣ���ͼ�������ھ���ȱ�ݣ�xֵС��1����֪NiXO����x=0.88�������߳�Ϊ4.28��10-10m ����֪��$\sqrt{2}$=1.4��
��֪NiXO���徧���ṹΪNaCl�ͣ���ͼ�������ھ���ȱ�ݣ�xֵС��1����֪NiXO����x=0.88�������߳�Ϊ4.28��10-10m ����֪��$\sqrt{2}$=1.4��