��Ŀ����
��1 mol I2��g�� ��2 mol H2����2L�ܱ������У���һ���¶��·�����Ӧ��I2��g�� + H2��g��  2HI��g�� ��H��0������ƽ�⡣HI���������w��HI����ʱ��仯��ͼ���ߣ�����ʾ��
2HI��g�� ��H��0������ƽ�⡣HI���������w��HI����ʱ��仯��ͼ���ߣ�����ʾ��
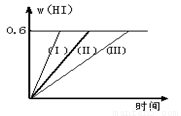
��1����ƽ��ʱ��I2��g�������ʵ���Ũ��Ϊmol/L ��
��2�����ı䷴Ӧ�������ڼ�������w��HI���ı仯�����ߣ��� ��ʾ������������w��HI���ı仯�����ߣ��� ��ʾ��������������� ���������������� ��������������������ţ�
�ٺ��������£������¶ȣ��ں��������£������¶ȣ��ۺ��������£���С��Ӧ����������ܺ��������£�����Ӧ����������ݺ��º��������£�������������
��3���������¶Ȳ��䣬����һ����ͬ��2L�ܱ������м���2mol I2��g����4mol H2��g��������Ӧ����ƽ��ʱ��HI����������� ��.��������ڡ����ڡ�С�ڣ���
��ϰ��ϵ�д�
�����Ŀ
1�����з�Ӧ�����ӷ���ʽ��д����ȷ���ǣ�������
| A�� | ��Ba��OH��2��Һ����μ���NH4HSO4��Һ���պó�����ȫ��Ba2++2OH-+H++SO42-+NH4+�TBaSO4��+NH3•H2O+H2O | |
| B�� | ������SO2����ͨ��NaClO��Һ�У�SO2+H2O+ClO�TSO42-+Cl-+2H+ | |
| C�� | ��Fe��NO3��3��Һ�м��������HI��Һ��Fe3++3NO3-+10I-+12H+�T5I2+Fe2++3NO��+6H2O | |
| D�� | NaHCO3��Һ�еμ���������ʯ��ˮ��2HCO3-+Ca2++2OH-�TCaCO3��+2H2O+CO32- |
2������˵������ȷ���ǣ�������
| A�� | ���ʵ����ǹ��ʵ�λ���е��߸�����������֮һ | |
| B�� | ���ʵ���ʵ���Ͼ������ʵ����� | |
| C�� | ����6.02��1023��ʾ����٤������ | |
| D�� | ����٤��������12g̼��������̼ԭ���� |
18���й��������ӷ���ʽ��˵����ȷ���ǣ�������
| A�� | ��NH4HCO3��Һ�мӹ�����NaOH��Һ�����ȣ�NH4++OH-$\frac{\underline{\;\;��\;\;}}{\;}$NH3��+H2O | |
| B�� | ��NaAlO2��Һ��ͨ��������CO2�ķ�ӦΪ��2AlO2-+CO2+3H2O=2Al��OH��3��+CO32- | |
| C�� | FeBr2��������Cl2��ӦΪ��2Fe2++2Br-+2Cl2=2Fe3++Br2+4Cl- | |
| D�� | ��Ũ�����ữ��KMnO4��Һ��H2O2��Ӧ��֤��H2O2���л�ԭ�ԣ�2MnO4-+6H++5H2O2=2Mn2++5O2��+8H2O |


 ������һ�ּ��߷�չDZ���������Դ��
������һ�ּ��߷�չDZ���������Դ��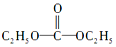 ����̼���µĽṹ��ʽΪ
����̼���µĽṹ��ʽΪ ��
��