��Ŀ����
ij��ѧ��ȤС��ⶨijFe2��SO4��3��Ʒ��ֻ������FeCl2���ʣ�����Ԫ�ص�����������������ʵ�鲽����в�����
�ٳ�ȡa g��Ʒ�������ձ��У�
�ڼ���50mL 1.0mol/Lϡ�����һ����������ˮ��ʹ��Ʒ�ܽ⣬Ȼ��ȷ���Ƴ�250.00mL��Һ��
����ȡ25.00mL���������õ���Һ�������ձ��У�������������ˮ��ʹ��Ӧ��ȫ��
�ܼ��������ˮ����ֽ��裬ʹ������ȫ��
�ݹ��ˣ�ϴ�ӳ�����
������ת�Ƶ�ij�����ڣ����ȡ����裬ֱ�������ɺ��ɫȫ����Ϊ����ɫ���ڸ���������ȴ�����º�����
�ߡ�

����������������ش�
��1����ͼ��ʾ�����У���ʵ�鲽��٢ڢ��б����õ���������E�� ������ĸ����
��2��������У�����50mL��1.0mol/LϡH2SO4��Ҫ98%���ܶ�1.84g/cm3����ŨH2SO4���Ϊ mL��
��3����Ʒ�е�����Fe2+�н�ǿ�Ļ�ԭ�ԣ���ɲ���ƽ���з�Ӧ�����ӷ���ʽ
Fe2++ ClO2+ = Fe3++ Cl-+ H2O
�������Ϸ���������ʵ����������ˮ��ΪClO2ʱ����ʵ������� �ƫ��ƫС����Ӱ�족���������ʵ�����ClO2��Cl2������Ч��֮��Ϊ ��
��4����������ڸ���������ȴ����ⶨ����Ԫ�ص����������� ���ƫ��ƫС����Ӱ�족�����ڢ��IJ����У�����������ȴ�����£�����������Ϊm1 g���ٴμ��Ȳ���ȴ�����³���������Ϊm2 g����m1��m2��ֵ�ϴ������IJ���Ӧ���� ��
�ٳ�ȡa g��Ʒ�������ձ��У�
�ڼ���50mL 1.0mol/Lϡ�����һ����������ˮ��ʹ��Ʒ�ܽ⣬Ȼ��ȷ���Ƴ�250.00mL��Һ��
����ȡ25.00mL���������õ���Һ�������ձ��У�������������ˮ��ʹ��Ӧ��ȫ��
�ܼ��������ˮ����ֽ��裬ʹ������ȫ��
�ݹ��ˣ�ϴ�ӳ�����
������ת�Ƶ�ij�����ڣ����ȡ����裬ֱ�������ɺ��ɫȫ����Ϊ����ɫ���ڸ���������ȴ�����º�����
�ߡ�

����������������ش�
��1����ͼ��ʾ�����У���ʵ�鲽��٢ڢ��б����õ���������E��
��2��������У�����50mL��1.0mol/LϡH2SO4��Ҫ98%���ܶ�1.84g/cm3����ŨH2SO4���Ϊ
��3����Ʒ�е�����Fe2+�н�ǿ�Ļ�ԭ�ԣ���ɲ���ƽ���з�Ӧ�����ӷ���ʽ
�������Ϸ���������ʵ����������ˮ��ΪClO2ʱ����ʵ�������
��4����������ڸ���������ȴ����ⶨ����Ԫ�ص�����������
���㣺̽�����ʵ���ɻ�������ʵĺ���,������ԭ��Ӧ����ʽ����ƽ
ר�⣺ʵ��̽�������ݴ�����
��������1�����ݸ�����������ѡȡ������
��2������������Һϡ���������ʵ����������������Һ�����
��3������������ԭ��Ӧ�����غ㡢ԭ���غ���ƽ���ӷ���ʽ����ԭ��һ������Ҫ�������������������������ƣ���ԭ��ʧ������һ�����������ı䲻Ӱ������������������������ǵõ�������֮�ȣ�
��4����������ڸ���������ȴ�������տ����е�ˮ����������������������ⶨ����Ԫ�ص���������ƫ�ߣ���������ֱ�������ɺ��ɫȫ����Ϊ����ɫ��������������صı������γ�������������0.1g��
��2������������Һϡ���������ʵ����������������Һ�����
��3������������ԭ��Ӧ�����غ㡢ԭ���غ���ƽ���ӷ���ʽ����ԭ��һ������Ҫ�������������������������ƣ���ԭ��ʧ������һ�����������ı䲻Ӱ������������������������ǵõ�������֮�ȣ�
��4����������ڸ���������ȴ�������տ����е�ˮ����������������������ⶨ����Ԫ�ص���������ƫ�ߣ���������ֱ�������ɺ��ɫȫ����Ϊ����ɫ��������������صı������γ�������������0.1g��
���
�⣺��1������ҩƷ����ƽ���Ȼ������Ȼ�������ˮ��Һ�������ԣ�����ȷ��ȡ25.00mL���������õ���Һ����ʽ�ζ��ܣ�����һ�����ʵ���Ũ�ȵ���Һ������ƿ���ʴ�Ϊ��CFG��
��2������50mL��1.0mol/LϡH2SO4��Ҫ98%���ܶ�1.84g/cm3����ŨH2SO4���Ϊ��ΪVml������ϡ��ǰ����Һ�������ʵ������䣬
=0.05L��1.0mol/L��V=2.7ml���ʴ�Ϊ��2.7��
��3����Ӧ�У��������ӱ仯Ϊ�����ӣ������������Ԫ�ػ��ϼ۴�+4�۱仯Ϊ-1�ۣ��仯5�ۣ�����ת����С������Ϊ5�����ݵ����غ��ԭ���غ���ƽ�õ����ӷ���ʽΪ��5Fe2++ClO2+4 H+�T5Fe3++Cl-+2H2O���������Ϸ���������ʵ����������ˮ��ΪClO2ʱ��ʵ����Ӱ�죬��ͬ������������ͬ��ͬ����ԭ�����ʱ�������Ҫʧȥ������ͬ�������ʵ�����ClO2��Cl2������Ч��֮��ΪΪת�Ƶ�����֮�ȣ�ClO2��Cl-��5e-��Cl2��2Cl-��2e-������1molClO2��Cl2������Ч��֮��Ϊ5��2��
�ʴ�Ϊ��5��1��4��H+��5��1��2����Ӱ�죻5��2��
��4����������ڸ���������ȴ�������տ����е�ˮ����������������������ⶨ����Ԫ�ص���������ƫ�ߣ���m1��m2��ֵ�ϴ��������ȣ���ȴ����������������ε����������0.1gΪֹ���ʴ�Ϊ�����������ȣ���ȴ����������������ε����������0.1gΪֹ��
��2������50mL��1.0mol/LϡH2SO4��Ҫ98%���ܶ�1.84g/cm3����ŨH2SO4���Ϊ��ΪVml������ϡ��ǰ����Һ�������ʵ������䣬
| Vml��1.84g/ml��98% |
| 98g/mol |
��3����Ӧ�У��������ӱ仯Ϊ�����ӣ������������Ԫ�ػ��ϼ۴�+4�۱仯Ϊ-1�ۣ��仯5�ۣ�����ת����С������Ϊ5�����ݵ����غ��ԭ���غ���ƽ�õ����ӷ���ʽΪ��5Fe2++ClO2+4 H+�T5Fe3++Cl-+2H2O���������Ϸ���������ʵ����������ˮ��ΪClO2ʱ��ʵ����Ӱ�죬��ͬ������������ͬ��ͬ����ԭ�����ʱ�������Ҫʧȥ������ͬ�������ʵ�����ClO2��Cl2������Ч��֮��ΪΪת�Ƶ�����֮�ȣ�ClO2��Cl-��5e-��Cl2��2Cl-��2e-������1molClO2��Cl2������Ч��֮��Ϊ5��2��
�ʴ�Ϊ��5��1��4��H+��5��1��2����Ӱ�죻5��2��
��4����������ڸ���������ȴ�������տ����е�ˮ����������������������ⶨ����Ԫ�ص���������ƫ�ߣ���m1��m2��ֵ�ϴ��������ȣ���ȴ����������������ε����������0.1gΪֹ���ʴ�Ϊ�����������ȣ���ȴ����������������ε����������0.1gΪֹ��
���������⿼����ʵ��̽��������ʵ��ⶨ����Һ���ƹ��̣���������������������ԭ��Ӧ��ƽ�ǽ���ؼ���ע��ʵ��ⶨ��Һ����ı仯����Ŀ�Ѷ��еȣ�

��ϰ��ϵ�д�
�����Ŀ
���з�Ӧ�����ӷ���ʽ��д��ȷ���ǣ�������
| A���ƺ���ˮ��Ӧ��Na+2H2O�TNa++2OH-+H2�� |
| B����������������������Һ��Al+2OH-�TAlO2-+H2�� |
| C����Al2��SO4��3��Һ�м�������İ�ˮ��Al3++3NH3?H2O=Al��OH��3��+3NH4+ |
| D������SO2ͨ��Ư����Һ�У�SO2+H2O+Ca2++2ClO-�TCaSO3��+2HClO |
2008��3��22���ǵ�16������ˮ�� ��World Water Day��������2008���ǹ��ʻ��������꣬���2008������ˮ�յ�����Ϊ����ˮ��������ˮ�������������зdz���Ҫ�����壬û��ˮ�Ͳ����������������ĵ������Ա���ˮ��Դ�����ͬ�����Σ����и��������ˮ��������Ⱦ����
��������ˮ�������ŷš��ں������ֵ�ԭ��й©����ˮ�����硡��ũҩ���ʵIJ�����ʹ�á���ʹ������ϴ�·ۣ�������
��������ˮ�������ŷš��ں������ֵ�ԭ��й©����ˮ�����硡��ũҩ���ʵIJ�����ʹ�á���ʹ������ϴ�·ۣ�������
| A��ֻ�Т٢ڢ� | B��ֻ�Т٢ڢ� |
| C��ֻ�Тۢ� | D��ֻ�Тڢۢ� |
�����£���0.1000mol/L��HCl��Һ�ζ�20.00mL 0.1000mol/L NH3?H2O��Һ���ζ���������ͼ������˵������ȷ���ǣ�������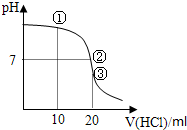

| A������Һ��c��C1-����c��NH4+����c��OH-����c��H+�� |
| B������Һ��c��NH4+��=c��C1-����c��OH-��=c��H+�� |
| C������Һ��c��H+����c��NH3?H2O����c��OH-�� |
| D���ζ������п��ܳ��֣�c��NH3?H2O����c��NH4+����c��OH-����c��Cl-����c��H+�� |
 ��ͼ��ͭпԭ��أ�ijͬѧ����ʵ����¼���£�
��ͼ��ͭпԭ��أ�ijͬѧ����ʵ����¼���£�

