��Ŀ����
��֪A��B��C��D��E��FΪ���ڱ�ǰ������ԭ�������������������Ԫ�أ�����A��Ԫ�����ڱ���ԭ�Ӱ뾶��С��Ԫ�أ�Bԭ���������������ڲ��������2����D��EΪͬ����Ԫ�أ���E��ԭ��������D��2����FԪ���ڵؿ��к���λ�ڽ���Ԫ�صĵڶ�λ���ش��������⣺
��1��FԪ�ؼ۲�����Ų�ʽΪ ��
��2������B2A2������˵������ȷ����
A��B2A2 �е�����ԭ�Ӷ�����8���ӽṹ
B��ÿ��B2A2������ �Ҽ��ͦм���Ŀ��Ϊ1��1
C��B2A2 ���ɼ��Լ��ͷǼ��Լ��γɵķǼ��Է���
D��B2A2�����е�A-B������s-sp�Ҽ�
��3��B��D�γɵ�һ����ԭ�ӷ�����C��D�γɵ�һ�ֻ����ﻥΪ�ȵ����壬����������������B��D�γɵĻ�����ĽṹʽΪ
��4��CԪ��ԭ�ӵĵ�һ�����ܱ�B��D��Ԫ��ԭ�ӵĵ�һ�����ܶ��ߵ� ��Ҫԭ����
��5��D���⻯���E���⻯��е�ߣ�����Ҫԭ���� ��E���⻯��ļ۲���ӶԻ���ģ��Ϊ ��Eԭ�ӵ��ӻ���ʽΪ ��
��6��F���ʵľ����ڲ�ͬ�¶��������ֶѻ���ʽ�������������ѻ������������ѻ����侧������߳��ֱ�Ϊa cm��b cm����F���ʵ������־����ܶ�֮��Ϊ ��Fԭ�ӵ���λ��֮��Ϊ ��
��1��FԪ�ؼ۲�����Ų�ʽΪ
��2������B2A2������˵������ȷ����
A��B2A2 �е�����ԭ�Ӷ�����8���ӽṹ
B��ÿ��B2A2������ �Ҽ��ͦм���Ŀ��Ϊ1��1
C��B2A2 ���ɼ��Լ��ͷǼ��Լ��γɵķǼ��Է���
D��B2A2�����е�A-B������s-sp�Ҽ�
��3��B��D�γɵ�һ����ԭ�ӷ�����C��D�γɵ�һ�ֻ����ﻥΪ�ȵ����壬����������������B��D�γɵĻ�����ĽṹʽΪ
��4��CԪ��ԭ�ӵĵ�һ�����ܱ�B��D��Ԫ��ԭ�ӵĵ�һ�����ܶ��ߵ� ��Ҫԭ����
��5��D���⻯���E���⻯��е�ߣ�����Ҫԭ����
��6��F���ʵľ����ڲ�ͬ�¶��������ֶѻ���ʽ�������������ѻ������������ѻ����侧������߳��ֱ�Ϊa cm��b cm����F���ʵ������־����ܶ�֮��Ϊ
���㣺�����ļ���,λ�ýṹ���ʵ����ϵӦ��
ר�⣺
��������֪A��B��C��D��E��FΪ���ڱ�ǰ������ԭ�������������������Ԫ�أ�����A��Ԫ�����ڱ���ԭ�Ӱ뾶��С��Ԫ�أ�AΪ��Ԫ�أ�Bԭ���������������ڲ��������2����B��2�����Ӳ㣬����������Ϊ4����BΪ̼Ԫ�أ�D��EΪͬ����Ԫ�أ���E��ԭ������ΪD��2����DΪ��Ԫ�أ�EΪ��Ԫ�أ���CΪ��Ԫ�أ�FԪ���ڵؿ��к���λ�ڽ���Ԫ�صĵڶ�λ��FΪFeԪ�أ�
��1�����ݺ�������Ų�������дFeԪ�ؼ۲�����Ų�ʽ��
��2��B2A2ΪC2H2��������Hԭ�Ӳ�����8���ӽṹ������2��C-H����1��C��C����������Ϊ�Ҽ��������к���1���Ҽ���2���м���C-HΪ���Լ���C��C�����ǷǼ��Լ���Ϊ�Գƽṹ�����ڷǼ��Է��ӣ�������Cԭ�Ӳ�ȡsp�ӻ�����C-H��Ϊs-sp�Ҽ���
��3��B��D�γɵ�һ����ԭ�ӷ�����C��D�γɵ�һ�ֻ����ﻥΪ�ȵ����壬��ΪCO2��N2O���ݴ˿�д���ṹʽ��
��4��NԪ��ԭ��2p�ܼ���3�����ӣ�Ϊ�����ȶ�״̬�������ϵͣ��ݴ��жϣ�
��5��D���⻯��ΪH2O������֮���������E���⻯��ΪH2S������֮��û���������H2S������Sԭ�ӳ�2��S-H��������2�Թµ��Ӷԣ��۲���Ӷ�Ϊ4���ݴ˴��⣻
��6�����������V=r3��rΪ�ⳤ�������㾧���к���Feԭ����Ŀ��V��=
��N����������Feԭ����Ŀ�����ݴ˼����ܶ�֮�ȣ�
�������������У��Զ����Fe��������֮�����Feԭ��λ�������ϣ�ÿ��Feԭ����Χ��12��Feԭ�ӣ��������������������ĵ�Fe��������֮�����Feԭ�Ӵ��ڶ����ϣ�ÿ��Feԭ����Χ��8��Feԭ�ӣ��ݴ˽��
��1�����ݺ�������Ų�������дFeԪ�ؼ۲�����Ų�ʽ��
��2��B2A2ΪC2H2��������Hԭ�Ӳ�����8���ӽṹ������2��C-H����1��C��C����������Ϊ�Ҽ��������к���1���Ҽ���2���м���C-HΪ���Լ���C��C�����ǷǼ��Լ���Ϊ�Գƽṹ�����ڷǼ��Է��ӣ�������Cԭ�Ӳ�ȡsp�ӻ�����C-H��Ϊs-sp�Ҽ���
��3��B��D�γɵ�һ����ԭ�ӷ�����C��D�γɵ�һ�ֻ����ﻥΪ�ȵ����壬��ΪCO2��N2O���ݴ˿�д���ṹʽ��
��4��NԪ��ԭ��2p�ܼ���3�����ӣ�Ϊ�����ȶ�״̬�������ϵͣ��ݴ��жϣ�
��5��D���⻯��ΪH2O������֮���������E���⻯��ΪH2S������֮��û���������H2S������Sԭ�ӳ�2��S-H��������2�Թµ��Ӷԣ��۲���Ӷ�Ϊ4���ݴ˴��⣻
��6�����������V=r3��rΪ�ⳤ�������㾧���к���Feԭ����Ŀ��V��=
| 56N |
| NA |
�������������У��Զ����Fe��������֮�����Feԭ��λ�������ϣ�ÿ��Feԭ����Χ��12��Feԭ�ӣ��������������������ĵ�Fe��������֮�����Feԭ�Ӵ��ڶ����ϣ�ÿ��Feԭ����Χ��8��Feԭ�ӣ��ݴ˽��
���
�⣺��֪A��B��C��D��E��FΪ���ڱ�ǰ������ԭ�������������������Ԫ�أ�����A��Ԫ�����ڱ���ԭ�Ӱ뾶��С��Ԫ�أ�AΪ��Ԫ�أ�Bԭ���������������ڲ��������2����B��2�����Ӳ㣬����������Ϊ4����BΪ̼Ԫ�أ�D��EΪͬ����Ԫ�أ���E��ԭ������ΪD��2����DΪ��Ԫ�أ�EΪ��Ԫ�أ���CΪ��Ԫ�أ�FԪ���ڵؿ��к���λ�ڽ���Ԫ�صĵڶ�λ��FΪFeԪ�أ�
��1��FeԪ����26��Ԫ�أ��۲�����Ų�ʽΪ3d64s2���ʴ�Ϊ��3d64s2��
��2��B2A2ΪC2H2��
A��������Hԭ�Ӳ�����8���ӽṹ����A����
B������2��C-H����1��C��C����������Ϊ�Ҽ��������к���1���Ҽ���2���м����Ҽ���м�֮��Ϊ3��2����B����
C��C-HΪ���Լ���C��C�����ǷǼ��Լ���Ϊ�Գƽṹ�����ڷǼ��Է��ӣ���C��ȷ��
D��������Cԭ�Ӳ�ȡsp�ӻ�����C-H��Ϊs-sp�Ҽ�����D��ȷ��
�ʴ�Ϊ��CD��
��3��B��D�γɵ�һ����ԭ�ӷ�����C��D�γɵ�һ�ֻ����ﻥΪ�ȵ����壬��ΪCO2��N2O��CO2��ֱ���ͣ���ṹʽΪO=C=O���ʴ�Ϊ��O=C=O��
��4��NԪ��ԭ��2p�ܼ���3�����ӣ�Ϊ�����ȶ�״̬�������ϵͣ����ȶ���ʧȥ��һ�ĵ�����Ҫ��������C��O�࣬�ʴ�Ϊ����ԭ�����������Ų�Ϊ2s22p3��2p3 Ϊ�����������ͽ��ȶ���ʧȥһ��������Ҫ����������̼������
��5��D���⻯��ΪH2O������֮���������E���⻯��ΪH2S������֮��û�������H2O���Ӽ���������H2S���Ӽ�ķ��»���ǿ�����Էе�ߣ���H2S������Sԭ�ӳ�2��S-H��������2�Թµ��Ӷԣ��۲���Ӷ�Ϊ4���۲���ӶԻ�������ģ��Ϊ�������ͣ�Sԭ�ӵ��ӻ���ʽΪSP3�ӻ���
�ʴ�Ϊ��H2O���Ӽ���������H2S���Ӽ�ķ��»���ǿ�������壻sp3��
��6���������������߳�Ϊacm�����V=a3������Feԭ����ĿΪ8��
+6��
=4����a3�ѣ����ģ�=4��
���������������߳�Ϊbcm�����V=b3������Feԭ����ĿΪ8��
+1=2����b3�ѣ����ģ�=2��
���ʦѣ����ģ����ѣ����ģ�=2b3��a3����������������ÿ��Feԭ����Χ��12��Feԭ�ӣ���������������ÿ��Feԭ����Χ��8��Feԭ�ӣ���Feԭ����λ��֮��Ϊ12��8=3��2���ʴ�Ϊ��2b3��a3��3��2��
��1��FeԪ����26��Ԫ�أ��۲�����Ų�ʽΪ3d64s2���ʴ�Ϊ��3d64s2��
��2��B2A2ΪC2H2��
A��������Hԭ�Ӳ�����8���ӽṹ����A����
B������2��C-H����1��C��C����������Ϊ�Ҽ��������к���1���Ҽ���2���м����Ҽ���м�֮��Ϊ3��2����B����
C��C-HΪ���Լ���C��C�����ǷǼ��Լ���Ϊ�Գƽṹ�����ڷǼ��Է��ӣ���C��ȷ��
D��������Cԭ�Ӳ�ȡsp�ӻ�����C-H��Ϊs-sp�Ҽ�����D��ȷ��
�ʴ�Ϊ��CD��
��3��B��D�γɵ�һ����ԭ�ӷ�����C��D�γɵ�һ�ֻ����ﻥΪ�ȵ����壬��ΪCO2��N2O��CO2��ֱ���ͣ���ṹʽΪO=C=O���ʴ�Ϊ��O=C=O��
��4��NԪ��ԭ��2p�ܼ���3�����ӣ�Ϊ�����ȶ�״̬�������ϵͣ����ȶ���ʧȥ��һ�ĵ�����Ҫ��������C��O�࣬�ʴ�Ϊ����ԭ�����������Ų�Ϊ2s22p3��2p3 Ϊ�����������ͽ��ȶ���ʧȥһ��������Ҫ����������̼������
��5��D���⻯��ΪH2O������֮���������E���⻯��ΪH2S������֮��û�������H2O���Ӽ���������H2S���Ӽ�ķ��»���ǿ�����Էе�ߣ���H2S������Sԭ�ӳ�2��S-H��������2�Թµ��Ӷԣ��۲���Ӷ�Ϊ4���۲���ӶԻ�������ģ��Ϊ�������ͣ�Sԭ�ӵ��ӻ���ʽΪSP3�ӻ���
�ʴ�Ϊ��H2O���Ӽ���������H2S���Ӽ�ķ��»���ǿ�������壻sp3��
��6���������������߳�Ϊacm�����V=a3������Feԭ����ĿΪ8��
| 1 |
| 8 |
| 1 |
| 2 |
| 56N |
| NA |
| 1 |
| 8 |
| 56N |
| NA |
����������Ŀ�ۺ��Խϴ��漰��������Ų����ɡ���ѧ�������Ӽ�����ռ�ṹ�������ܡ��۲���ӶԻ������ۡ��ӻ�������ۡ�����ʽ���������㣬�Ƕ����ʽṹ���ۺϿ��飬�Ѷ��еȣ���Ҫ��֪ʶȫ���������⣮

��ϰ��ϵ�д�
 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
�����Ŀ
���и������У�����ԭ�Ӷ����������8���ӽṹ���ǣ�������
| A��BeCl2 |
| B��PCl3 |
| C��NH3 |
| D��PCl5 |
��֪ijԪ�ص�ǰ8�������ܣ�I1/kJ?mol-1����I1=577��I2=1820��I3=2740��I4=11600��I5=14800��I6=18400��I7=23400��I8=27500����Ԫ��ԭ�ӵ�������м������ӣ���������
| A��1�� | B��2�� | C��3�� | D��4�� |
����ʵ�鷽�����е��ǣ�������
| A���ڵ�����Һ�м�ϡ�����ַ�Ӧ���ټ���������Һ���ɼ�����۵�ˮ����� |
| B������ˮ���Լ��𱽡��Ҵ������Ȼ�̼����Һ�� |
| C����ȥ���еı��ӣ��ɼ���NaOH��Һ���ٷ�Һ |
| D������������NaOH���Ҵ���Һ���ȣ�������������ֱ��ͨ�����Ը��������Һ�У���Һ��ɫ��֤�������鷢������ȥ��Ӧ�� |

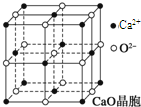 ���������з�Ӧ�ϳ������谷��CaO+3C
���������з�Ӧ�ϳ������谷��CaO+3C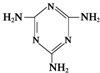 �׳ơ����������������������谷����������
�׳ơ����������������������谷����������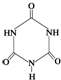 �����������������谷�����֮��ͨ��
�����������������谷�����֮��ͨ��