��Ŀ����
19�����Ϊ��ʽ���ڱ���һ���֣����еı�Ŵ�����Ӧ��Ԫ�أ�
��ش��������⣺
��1����ѧ�ҷ��֣��ڡ��ܡ�������Ԫ�ص�ԭ���γɵľ�����г����ԣ��侧���Ľṹ�ص���ͼ��ʾ��ͼ�Тڡ��ܡ���ֱ�λ�ھ��������ġ����㡢���ģ�����û�����Ļ�ѧʽΪMgNi3C���ö�Ӧ��Ԫ�ط��ű�ʾ����
��2��ijԪ�صļ۵����Ų�ʽΪnsnnpn+1����Ԫ����Ԫ�آ��γɵ�18���ӵ�X���ӵĽṹʽΪ
 ����Ԫ�ػ�����Ԫ�آ��γ�10���ӵ��������Y����������Y����ͨ��ʢ�к���Ԫ�ص���������Һ�У���Ӧ�����е�ʵ������Ϊ�Ȳ�����ɫ������Ȼ������ܽ⣬�������ɫ��Һ��
����Ԫ�ػ�����Ԫ�آ��γ�10���ӵ��������Y����������Y����ͨ��ʢ�к���Ԫ�ص���������Һ�У���Ӧ�����е�ʵ������Ϊ�Ȳ�����ɫ������Ȼ������ܽ⣬�������ɫ��Һ����3���ȽϢۣ��ݣ��ޣ��ߣ�������Ԫ�صĵ縺�Դ�С���ɴ�С���е�˳��ΪCa��Al��S��Cl��O���� Ԫ�ط��ű�ʾ����
��4����Ԫ�ص��ʾ����ȡ�ѻ���ʽ�����������ܶѻ����ռ�������Ϊ74%��
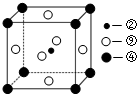
���� ��Ԫ�����ڱ����Եó�Ԫ�آ�ΪH����ΪC����ΪO����ΪMg����ΪMn����ΪS����ΪCl����ΪCa����ΪNi����ΪCu��
��1���ڡ��ܡ���ֱ�λ�ھ��������ġ����㡢���ģ�Cԭ�Ӹ���Ϊ1��Mgԭ�Ӹ���Ϊ8��$\frac{1}{8}$=1��Niԭ�Ӹ���Ϊ6��$\frac{1}{2}$=3��
��2��Ԫ�ص����������Ų�ʽΪnsnnpn+1����n=2���ʸ�Ԫ�ص����������Ų�ʽΪ2s22p3��ΪNԭ�ӣ�Ԫ����Ԫ�آ��γɵ�18���ӵ�X����ΪN2H4��Ԫ�آ�ΪHԪ�أ���X�γɵ�����ΪNH3��������ͭ��Һ��������ɫ���������γ�����
��3���ǽ�����Խǿ���縺��Խ��
��4��Cu���ʵľ���ѻ���ʽΪ���������ܶѻ������þ�̯�����㾧����Cuԭ����Ŀ����Cuԭ�Ӱ뾶Ϊr�����ⳤΪ4r��$\frac{\sqrt{2}}{2}$=2$\sqrt{2}$r���ռ�������=$\frac{������ԭ�������}{�������}$��100%��
��� �⣺��Ԫ�����ڱ����Եó�Ԫ�آ�ΪH����ΪC����ΪO����ΪMg����ΪMn����ΪS����ΪCl����ΪCa����ΪNi����ΪCu��
��1���ڡ��ܡ���ֱ�λ�ھ��������ġ����㡢���ģ�Cԭ�Ӹ���Ϊ1��Mgԭ�Ӹ���Ϊ8��$\frac{1}{8}$=1��Niԭ�Ӹ���Ϊ6��$\frac{1}{2}$=3����ѧʽΪMgNi3C���ʴ�Ϊ��MgNi3C��
��2��Ԫ�ص����������Ų�ʽΪnsnnpn+1����n=2���ʸ�Ԫ�ص����������Ų�ʽΪ2s22p3��ΪNԭ�ӣ�Ԫ����Ԫ�آ��γɵ�18���ӵ�X����ΪN2H4����ṹʽΪ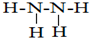 ��Ԫ�آ�ΪHԪ�أ���X�γɵ�����ΪNH3��������ͭ��Һ��������ɫ���������γ�������۲쵽������Ϊ�Ȳ�����ɫ������Ȼ������ܽ⣬�������ɫ��Һ��
��Ԫ�آ�ΪHԪ�أ���X�γɵ�����ΪNH3��������ͭ��Һ��������ɫ���������γ�������۲쵽������Ϊ�Ȳ�����ɫ������Ȼ������ܽ⣬�������ɫ��Һ��
�ʴ�Ϊ��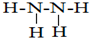 ���Ȳ�����ɫ������Ȼ������ܽ⣬�������ɫ��Һ��
���Ȳ�����ɫ������Ȼ������ܽ⣬�������ɫ��Һ��
��3���ǽ�����Խǿ���縺��Խ����縺�Դ�С����˳����Ca��Al��S��Cl��O���ʴ�Ϊ��Ca��Al��S��Cl��O��
��4��Cu���ʵľ���ѻ���ʽΪ���������ܶѻ���������Cuԭ����ĿΪ8��$\frac{1}{8}$+6��$\frac{1}{2}$=4����Cuԭ�Ӱ뾶Ϊr������Cuԭ�������Ϊ4��$\frac{4}{3}$��r3����Cuԭ�Ӱ뾶Ϊr�����ⳤΪ4r��$\frac{\sqrt{2}}{2}$=2$\sqrt{2}$r���������Ϊ��2$\sqrt{2}$r��3=16$\sqrt{2}$r3���ռ�������=$\frac{\frac{16}{3}��{r}^{3}}{16\sqrt{2}{r}^{3}}$��100%=74%��
�ʴ�Ϊ�������������ܶѻ���74%��
���� �����ۺϿ������ʽṹ�����ʣ�Ϊ��Ƶ���㣬�漰Ԫ�����ڱ�������������ṹ����㣬�Ƕ�ѧ���ۺ������Ŀ��飬��4��Ϊ�����ѵ㣬��Ҫѧ���߱�һ���Ŀռ���������ѧ������������Ŀ�Ѷ��еȣ�

 ��У����ϵ�д�
��У����ϵ�д�| X | |||
| Y | X | R | |
| W |
| A�� | ����Ԫ�ؿ��ܶ��ǽ���Ԫ�� | |
| B�� | ����Ԫ�ص�ԭ������������һ��������2 | |
| C�� | X���⻯��ķе�һ����Z���⻯��ķе�� | |
| D�� | R������������Ӧ��ˮ����һ����ǿ�� |
| A�� | MgCl2 | B�� | KNO3 | C�� | NaCl | D�� | ��NH4��2SO4 |
| A�� | ��ѹ | B�� | ���� | C�� | ��ѹ | D�� | ����B��Ũ�� |
| A�� | ��ͬ���ʵ���Ũ�ȵ�FeI2��Һ��Br2ˮ��Һ�������ϣ�2Fe2++2I-+2Br2�T2Fe3++I2+4Br- | |
| B�� | ��Ba��OH��2��Һ�м������NH4HSO4��Һ��NH4++Ba2++2OH-+H++SO42-�TBaSO4��+NH3•H2O+H2O | |
| C�� | ��Ư����Һ��ͨ�������SO2��Ca2++2ClO-+SO2+H2O�TCaSO3��+2HClO | |
| D�� | ���������������������Һ�У�Fe3O4+8H++2I-�T3Fe2++I2+4H2O |
��1��������һ������Ľྻȼ�ϣ���֪��
CH4��g��+2O2��g���TCO2��g��+2H2O��g������H=-802.3kJ•mol-1
H2O��1���TH2O��g������H=+44.0kJ•mol-l
��4.8g����������ȫȼ������Һ̬ˮ���ų�������Ϊ267.1kJ��
��2�����ü�����ˮ��Ӧ�Ʊ���������ԭ�����ۣ������ƹ��ֵ��
�÷�ӦΪCH4��g��+H2O��g��?CO��g��+3H2��g������H=+206.1kJ•mol-l��
����800��ʱ����Ӧ�Ļ�ѧƽ�ⳣ��K=l.0��ijʱ�̲�ø��¶����ܱ������и����ʵ����ʵ���Ũ�����±���
| CH4 ��g�� | H2O ��g�� | CO ��g�� | H2 ��g�� |
| 3.0mol•L-1 | 8.5mol•L-1 | 2.0mol•L-1 | 2.0mol•L-1 |
a��v��������v���棩 b��v��������v���棩
c��v������=v���棩 d�����ж�
��Ϊ��̽���¶ȡ�ѹǿ��������ѧ��Ӧ���ʵ�Ӱ�죬ijѧϰС���������������Ա�ʵ�飨�¶�Ϊ360���480�桢ѹǿΪ101kPa��303kPa������ʵ���������±�����
| ʵ����� | �¶�/�� | ѹǿ/kPa | V��CH4��/mol•L-1•s-1 | V��H2O��/mol•L-1•s-1 |
| 1 | 360 | P1 | 0.100 | 0.100 |
| 2 | 480 | 101 | 0.120 | 0.120 |
| 3 | 360 | P2 | 0.080 | 0.080 |
ʵ��2��3��Ŀ����̽���¶ȶԻ�ѧ��Ӧ���ʵ�Ӱ�죮
ʵ��1��2��3�з�Ӧ�Ļ�ѧƽ�ⳣ���Ĵ�С��ϵ��K1=K3��K2 ����K1��K2��K3��ʾ����
 X����Ԫ���γɵĻ�����ĵ���ʽ��[H��]-Ca2+[��H]-��
X����Ԫ���γɵĻ�����ĵ���ʽ��[H��]-Ca2+[��H]-��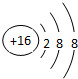 ��D��E���γ�һ�ַ��ӣ��÷��ӵĽṹʽΪS=C=S��D������Ԫ�ص��⻯���У��е���͵������⣨�����ƣ���X��E�γɵ����ӻ����� XE2���У���С����ޡ������ۼ���
��D��E���γ�һ�ַ��ӣ��÷��ӵĽṹʽΪS=C=S��D������Ԫ�ص��⻯���У��е���͵������⣨�����ƣ���X��E�γɵ����ӻ����� XE2���У���С����ޡ������ۼ���