��Ŀ����
11����Դ���������Ϊ��Լ������ᾭ�÷�չ��ƿ����Խ��Խ��Ĺ��ҿ�ʼʵ�С�����ƻ���������̫������Դ��Ѱ�÷�չ���¶�������1��̫������ˮ���г�ʹ��һ�����������Ͻ������Ϊ���ռ���̫��������Ϳ�㣬��̬��ԭ��M���ϵ�δ�ɶԵ�����Ϊ2��
��2�������ѧ�����������л�̫���ܹ�����Ч��ͻ��5.3%�����ߴ���C60���䡰������������C60�Ľṹ��ͼ1��������̼ԭ�ӹ�����ӻ�����Ϊsp2��1mol C60�����Цм�����ĿΪ30NA��
��3������̪ݼ������ڹ�̫���ܵ��������Ҫ���ã�һ�ֽ���þ̪ݼ�����Ľṹ��ͼ2���ýṹ�У�̼��֮��Ĺ��ۼ������ЦҼ����м�����ԭ�ӹ���ص���ʽ��д���ۼ������ͣ�������ͼ2���ü�ͷ��ʾ����λ����
��4����Ԫ�����ﱡĤ̫���ܵ�ز���Ϊ���Σ�����Ҫ�����黯�ء����ӡ���п��ͭ������Ĥ��صȣ�
�ٵ�һ�����ܣ�As��Se�����������������=������
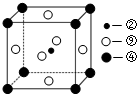
����п�ľ����У��ṹ��ͼ3��ʾ���������ӵ���λ����4��
�۶����������ӵĿռ乹��ΪV�Σ�
���黯�ؿ��ɣ�CH3��3Ga��AsH3��700���·�Ӧ�Ƶã���Ӧ�ķ���ʽΪ��CH3��3Ga+AsH3$\frac{\underline{\;700��\;}}{\;}$GaAs+3CH4��
���� ��1����̬Niԭ�Ӻ�������Ų�ʽΪ1S22S22P63S23P63d84s2���ݴ��жϣ�
��2��ÿ��Cԭ�ӳ�3���Ҽ���û�йµ��Ӷԣ��ӻ������ĿΪ3���ݴ�ȷ���ӻ���ʽ��
���þ�̯������ÿ��̼ԭ�Ӻ��м����м����Ӷ�����1mol C60�����Цм�����Ŀ��
��3��C��Nԭ��֮���γ�˫������������λ�����ṩ�µ��ӶԵ�ԭ��ָ���ṩ�չ����ԭ�ӣ�
��4����ͬһ����Ԫ�صĵ�һ����������ԭ�����������������������ƣ�ע���VA��Ԫ�ش���ͬ��������Ԫ�صĵ�һ�����ܣ�
���ɾ����ṹ��֪��ÿ������������4��п���ӣ�������λ����4��
�۸��ݼ۲���ӶԻ�������ȷ����ռ乹�ͣ�
���黯�ؿ��ɣ�CH3��3Ga��AsH3��700���·�Ӧ�Ƶã���Ԫ���غ��֪�����ɼ��飮
��� �⣺��1����̬Niԭ�Ӻ�������Ų�ʽΪ1S22S22P63S23P63d84s2��3d�����2�������ӣ��ʴ�Ϊ��2��
��2��ÿ��Cԭ�ӳ�3���Ҽ���û�йµ��Ӷԣ��ӻ������ĿΪ3������Cԭ�Ӳ���sp2 �ӻ���
ÿ��̼ԭ�Ӻ��еĦм�����Ϊ$\frac{1}{2}$������1mol C60�����Цм�����Ŀ=1mol��60��$\frac{1}{2}$��NAmol-1=30NA��
�ʴ�Ϊ��sp2��30NA��
��3��C��Nԭ��֮���γ�˫��������������Ϊ�Ҽ���˫������1���Ҽ���1���м���
��λ�����ṩ�µ��ӶԵ�ԭ��ָ���ṩ�չ����ԭ�ӣ����Ը�������е���λ��Ϊ�� ��
��
�ʴ�Ϊ���Ҽ����м��� ��
��
��3����As��Se����ͬһ���ڣ���As���ڵ�VA�壬Se���ڵ�VIA�壬Asԭ��4p�ܼ�����3�����ӣ�Ϊ�����ȶ�״̬�������ϵͣ���һ�����ܸ���Se�����Ե�һ������As��Se��
�ʴ�Ϊ������
���ɾ����ṹ��֪��ÿ������������4��п���ӣ�������λ����4��
�ʴ�Ϊ��4��
�۶�������������Seԭ�Ӽ۲���Ӷ�=2+$\frac{6-2��2}{2}$=3���Һ���һ���µ��Ӷԣ�������ռ�ṹΪV�Σ�
�ʴ�Ϊ��V�Σ�
���黯�ؿ��ɣ�CH3��3Ga��AsH3��700���·�Ӧ�Ƶã���Ԫ���غ��֪�����ɼ��飬��Ӧ����ʽΪ����CH3��3Ga+AsH3$\frac{\underline{\;700��\;}}{\;}$GaAs+3CH4 ��
�ʴ�Ϊ����CH3��3Ga+AsH3$\frac{\underline{\;700��\;}}{\;}$GaAs+3CH4��
���� ���⿼�������ʽṹ�����ʣ��漰��������Ų����ӻ���������ӽṹ�����ʡ������ܡ���ѧ���������ȣ���Ŀ�Ѷ��еȣ�ע����λ���γ������������ڿ���ѧ���Ի���֪ʶ���ۺ�Ӧ��������

 ����ͼ���������������ϵ�д�
����ͼ���������������ϵ�д� ����ѧҵ���Ե�����ϵ�д�
����ѧҵ���Ե�����ϵ�д�| A�� | ����ͼ��� | B�� | CH3COOH��C3H6O2 | C�� |  �� �� | D�� | C2H4��C4H8 |
| A�� | H2��D2��Ϊͬλ�� | |
| B�� | �������еĻ�ѧ����Ϊ�Ǽ��Թ��ۼ� | |
| C�� | NH4Cl�ĵ���ʽ�� | |
| D�� | S2-�Ľṹʾ��ͼ�� |
 ������ͬѧ�ֱ�Ժ�+4����Ԫ�ص��������ʽ�����̽����
������ͬѧ�ֱ�Ժ�+4����Ԫ�ص��������ʽ�����̽������1��������ͼװ�ý���ʵ�飨�������Ѽ��飬���Ⱥͼг�װ������ȥ����ʵ�����һ��ʱ���C��D�ж��������Եİ�ɫ����������
���ΪBaSO4��
��A�з�Ӧ�Ļ�ѧ����ʽ��Cu+2H2SO4��Ũ��$\frac{\underline{\;\;��\;\;}}{\;}$CuSO4+SO2��+2H2O��
��Ϊ̽��SO2��D���������ķ�Ӧ����һ��ʵ�鷢�֣����ְ�ɫ�����Ĺ����У�D��Һ��NO3-Ũ�ȼ������䣮�ݴ˵ó����ۣ�D�г��ְ�ɫ��������Ҫԭ�������������£���+4����Ԫ�����ʣ�SO2��H2SO3����O2��������SO42-��
��2����������ʵ��Ժ�+4����Ԫ�ص��������ʼ�������̽����
| ��� | ʵ����� | ʵ������ |
| 1 | ȡ0.3g ����Na2SO3���壬�����м���10mL 2mol•L-1 ���ᣬ�ٵ���4��BaCl2��Һ | ������ɫ���ݣ�����BaCl2��Һ��ʼ������4min����Һ����� |
| 2 | ȡ0.3g ����Na2SO3���壬�����м���10mL 2mol•L-1 HNO3���ٵ���4��BaCl2��Һ | ������ɫ���ݣ�����BaCl2��Һ��ʼ������2h����Һ����� |
| 3 | ȡ0.3g ����Na2SO3���壬�����м���10mL ŨHNO3���ٵ���4��BaCl2��Һ | ��������ɫ���壻����BaCl2��Һ����Һ��������������ɫ���� |
����ʵ��1��2��3�Աȣ����Եõ����ۣ���+4����Ԫ�����ʿɱ�O2��ŨHNO3������
����ͨ���������Ϸ��֣�Na+��ʵ��1��2�г��ֻ��ǵ�ʱ����Ӱ�죬���ǽ�һ��̽��Cl-��NO3-�����Ӱ�죺
| ��� | ʵ����� | ʵ������ |
| 4 | ȡ0.3g����Na2SO3��1.17gNaCl������������м���10mL 2mol•L-1 HNO3���ٵ���4��BaCl2��Һ | ������ɫ���ݣ�����BaCl2��Һ��ʼ������20min����Һ����� |
ii��ʵ��1��4�Աȣ��һ�����ۣ�NO3-�Ĵ��ڿ��Լ�����Һ��+4����Ԫ�ص�������
��ͨ������ʵ�飬��ͬѧ��Ϊ��ȷ��ij��Һ�к���SO42-��ʵ�鷽����ȡ����Һ���������ȵμ�bd������ĸ��ţ���
a.2mol•L-1���ᣬ�ٵμ�BaCl2��Һ��һ��ʱ�����ְ�ɫ����
b.2mol•L-1���ᣬ�ٵμ�BaCl2��Һ���������ְ�ɫ����
c.2mol•L-1���ᣬ�ٵμ�BaCl2��Һ��һ��ʱ�����ְ�ɫ����
d.2mol•L-1���ᣬ�ٵμ�BaCl2��Һ���������ְ�ɫ������
| A�� | X��W���������֮��Ϊ11 | |
| B�� | Y���⻯����ȶ��Ա�W��ǿ | |
| C�� | ԭ�Ӱ뾶�ɴ�С��˳��ΪW��Z��Y��X | |
| D�� | Y�ֱ���X��Z�γɵĻ������л�ѧ��������ͬ |

| A�� | ���ֵ�����Һ�ͼ�������Һ | B�� | ����������Ҵ� | ||
| C�� | ��������ˮ�붹�� | D�� | �������������뱥��Na0H��Һ |
| A�� | 1��8 | B�� | 6��8 | C�� | 16��8 | D�� | 12��17 |





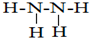 ����Ԫ�ػ�����Ԫ�آ��γ�10���ӵ��������Y����������Y����ͨ��ʢ�к���Ԫ�ص���������Һ�У���Ӧ�����е�ʵ������Ϊ�Ȳ�����ɫ������Ȼ������ܽ⣬�������ɫ��Һ��
����Ԫ�ػ�����Ԫ�آ��γ�10���ӵ��������Y����������Y����ͨ��ʢ�к���Ԫ�ص���������Һ�У���Ӧ�����е�ʵ������Ϊ�Ȳ�����ɫ������Ȼ������ܽ⣬�������ɫ��Һ��