��Ŀ����
��ͼ1Ϊϸ��ұͭ�ͻ�ұﴵ���Ҫ���̣�
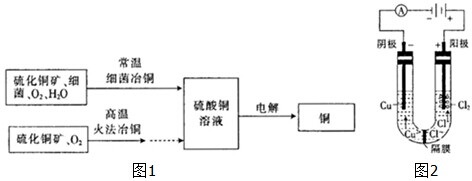
��1������ͭ��Һһ��� ����ᡱ��������С����ԣ�ԭ����
�������ӷ���ʽ��ʾ����д���������ͭ��Һ�Ļ�ѧ����ʽ�� ���������У�ʼ����������������
��2��ϸ��ұ���ֳ���������ǽ���ʪ��ұ��ҵ�ϵ�һ���¹��գ�ϸ��ұͭ���ұͭ��ȣ��ŵ�Ϊ ��д��һ�㼴�ɣ���
��3���ö��Ե缫�ֱ���Ũ���Ȼ�ͭ��Һ������ͭ��Һ�����Ũ���Ȼ�ͭ��Һʱ���� �����н���ͭ���ɣ�ͬʱ��������������غ�ɫ��Һ�����������ͭ��Һʱ��û���غ�ɫ��Һ���ɣ���ͼ2�ǹ����غ�ɫ��Һ�ɷֵ�̽����
����ͬѧ��Ϊ�������������ֵ��غ�ɫ��Һ��������Ӧ�Ľ��������Ϊ���IJ²��� ����ȷ�� �����ȷ������ȷ������ԭ����
����1��һ����л�ϼ�̬��ָ��������ͬһԪ�ش������ֲ�ͬ�Ļ��ϼۣ���Fe3O4�е� FeԪ���������ʵ���ɫ�ȵ�һ��̬�����ʵ���ɫҪ�
����2��CuCl����ˮ��������Ũ���ᣮ
�ڲ��룺�غ�ɫ��Һ�п��ܺ��ӵ������� ����3����Ҫ���ӷ��ţ���
����֤���룺���ʵ�鷽���������غ�ɫ��Һ����
ȡ���� �������Թ��У����� ʹ���ܽ⣬�ټ��� ��Һ���۲�����
����֪���ǰ��U�ι��м����� 100 mLO.5mol��L-1 CuCl2��Һ������cʱ��·��һ��ת����0.03mol���ӣ�����������0.64gͭ�����γɵĵͼ������ӵ����ʵ���Ϊ mol��

��1������ͭ��Һһ���
�������ӷ���ʽ��ʾ����д���������ͭ��Һ�Ļ�ѧ����ʽ��
��2��ϸ��ұ���ֳ���������ǽ���ʪ��ұ��ҵ�ϵ�һ���¹��գ�ϸ��ұͭ���ұͭ��ȣ��ŵ�Ϊ
��3���ö��Ե缫�ֱ���Ũ���Ȼ�ͭ��Һ������ͭ��Һ�����Ũ���Ȼ�ͭ��Һʱ���� �����н���ͭ���ɣ�ͬʱ��������������غ�ɫ��Һ�����������ͭ��Һʱ��û���غ�ɫ��Һ���ɣ���ͼ2�ǹ����غ�ɫ��Һ�ɷֵ�̽����
����ͬѧ��Ϊ�������������ֵ��غ�ɫ��Һ��������Ӧ�Ľ��������Ϊ���IJ²��� ����ȷ��
����1��һ����л�ϼ�̬��ָ��������ͬһԪ�ش������ֲ�ͬ�Ļ��ϼۣ���Fe3O4�е� FeԪ���������ʵ���ɫ�ȵ�һ��̬�����ʵ���ɫҪ�
����2��CuCl����ˮ��������Ũ���ᣮ
�ڲ��룺�غ�ɫ��Һ�п��ܺ��ӵ�������
����֤���룺���ʵ�鷽���������غ�ɫ��Һ����
ȡ����
����֪���ǰ��U�ι��м����� 100 mLO.5mol��L-1 CuCl2��Һ������cʱ��·��һ��ת����0.03mol���ӣ�����������0.64gͭ�����γɵĵͼ������ӵ����ʵ���Ϊ
���㣺����ұ����һ��ԭ��,���ԭ��
ר�⣺�绯ѧר��,Ԫ�ؼ��仯����
��������1������ͭΪǿ�������Σ�ˮ������ԣ����Ե缫���һ����������ͭ��Һ������������Cu2++2e-�TCu����������Һ�е����������ӷŵ磬4OH--4e-�TH2O+O2����
��2������Դ�������ȷ���������ϸ��ұͭ���ŵ㣻
��3���ö��Ե缫����Ȼ�ͭ��Һ����һ�Σ���������������Ӧ��2Cl--2e-=Cl2��������������ԭ��Ӧ��Cu2++2e-=Cu���ڶ��Σ�2H2O
2H2��+O2�����������ͭ��Һʱ��һ�Σ�2CuSO4+2H2O
2Cu��+O2��+2H2SO4���ڶ��Σ�2H2O
2H2��+O2����
�ٵ��Ũ���Ȼ�ͭ��Һ���ڶ��ε��ˮ���������������������������������������
�ڸ��ݻ�ϼ�̬�����ʵ���ɫ�ȵ�һ��̬�����ʵ���ɫ�CuCl����ˮ��������Ũ����������
�۸���CuCl��CuCl2�����ʷ������
�����õ����غ㷨�������0.64gͭת��0.02mol���ӣ�����0.01mol����Ϊ+2��ͭ���ӵõ�������+1�۵�ͭ���ӣ��ݴ˷������
��2������Դ�������ȷ���������ϸ��ұͭ���ŵ㣻
��3���ö��Ե缫����Ȼ�ͭ��Һ����һ�Σ���������������Ӧ��2Cl--2e-=Cl2��������������ԭ��Ӧ��Cu2++2e-=Cu���ڶ��Σ�2H2O
| ||
| ||
| ||
�ٵ��Ũ���Ȼ�ͭ��Һ���ڶ��ε��ˮ���������������������������������������
�ڸ��ݻ�ϼ�̬�����ʵ���ɫ�ȵ�һ��̬�����ʵ���ɫ�CuCl����ˮ��������Ũ����������
�۸���CuCl��CuCl2�����ʷ������
�����õ����غ㷨�������0.64gͭת��0.02mol���ӣ�����0.01mol����Ϊ+2��ͭ���ӵõ�������+1�۵�ͭ���ӣ��ݴ˷������
���
�⣺��1������ͭΪǿ�������Σ�ˮ��Cu2++2H2O?Cu��OH��2+2H+�����ԣ��ö��Ե缫�������ͭ��Һ���������У�ʼ������������������������Cu2++2e-�TCu���������������ӷŵ磬4OH--4e-�TH2O+O2�����ܷ�ӦΪ��2CuSO4+2H2O
2Cu��+O2��+2H2SO4��
�ʴ�Ϊ���Cu2++2H2O?Cu��OH��2+2H+��2CuSO4+2H2O
2Cu��+O2��+2H2SO4��
��2��ʪ����ͭ������Һ�н��У����ʪ����ͭ��ϸ��ұͭ���ڳ�����������ұͭ����Լ��Դ���������豸���������㣻�����������ơ�Ͷ���١��ɱ��ͣ����˴���¯����
�ʴ�Ϊ����Լ��Դ���������豸���������㣻�����������ơ�Ͷ���١��ɱ��ͣ����˴���¯����
��3���ö��Ե缫����Ȼ�ͭ��Һ����һ�Σ���������������Ӧ��2Cl--2e-=Cl2��������������ԭ��Ӧ��Cu2++2e-=Cu���ڶ��Σ�2H2O
2H2��+O2�����������ͭ��Һʱ��һ�Σ�2CuSO4+2H2O
2Cu��+O2��+2H2SO4���ڶ��Σ�2H2O
2H2��+O2����
���ö��Ե缫����Ȼ�ͭ��Һ�����������������������ƶ���������������һ�Σ�Cu2++2e-=Cu���ڶ��Σ�2H++2e-=H2������������������������������������������غ�ɫ��Һ��������������Ӧ�Ľ����
�ʴ�Ϊ������ȷ�������������������
�ڸ��������Ϣ����ϼ�̬�����ʵ���ɫ�ȵ�һ��̬�����ʵ���ɫ�ͭ��Cu2+��Cu+�����ӣ������غ�ɫ��Һ�п��ܺ��е�������Cu2+��Cu+����Һ�л����ܴ���������Cl-��
�ʴ�Ϊ��Cu2+��Cu+��Cl-��
��Ϊ����֤��Һ���Ƿ����Cu2+��Cu+����ͨ���������������֤��ȡ�����Ȼ���ͭ���������Թ��У�����Ũ����ʹ���ܽ⣬�ټ����Ȼ�ͭ��Һ���۲�����
�ʴ�Ϊ���Ȼ���ͭ��Ũ����Ȼ�ͭ��
��100mL 0.5mol?L-1 CuCl2��Һ�к�0.05molCuCl2��Cu2++2e-=Cu������0.64gͭת��0.02mol���ӣ�����cʱ��·��һ��ת����0.03mol���ӣ����������Ϣ����ϼ�̬�����ʵ���ɫ�ȵ�һ��̬�����ʵ���ɫ����Ի���0.01mol����Ϊ+2��ͭ���ӵõ�������+1�۵�ͭ���ӣ�Cu2++e-=Cu+�����γɵĵͼ������ӵ����ʵ���Ϊ0.01mol��
�ʴ�Ϊ��0.01��
| ||
�ʴ�Ϊ���Cu2++2H2O?Cu��OH��2+2H+��2CuSO4+2H2O
| ||
��2��ʪ����ͭ������Һ�н��У����ʪ����ͭ��ϸ��ұͭ���ڳ�����������ұͭ����Լ��Դ���������豸���������㣻�����������ơ�Ͷ���١��ɱ��ͣ����˴���¯����
�ʴ�Ϊ����Լ��Դ���������豸���������㣻�����������ơ�Ͷ���١��ɱ��ͣ����˴���¯����
��3���ö��Ե缫����Ȼ�ͭ��Һ����һ�Σ���������������Ӧ��2Cl--2e-=Cl2��������������ԭ��Ӧ��Cu2++2e-=Cu���ڶ��Σ�2H2O
| ||
| ||
| ||
���ö��Ե缫����Ȼ�ͭ��Һ�����������������������ƶ���������������һ�Σ�Cu2++2e-=Cu���ڶ��Σ�2H++2e-=H2������������������������������������������غ�ɫ��Һ��������������Ӧ�Ľ����
�ʴ�Ϊ������ȷ�������������������
�ڸ��������Ϣ����ϼ�̬�����ʵ���ɫ�ȵ�һ��̬�����ʵ���ɫ�ͭ��Cu2+��Cu+�����ӣ������غ�ɫ��Һ�п��ܺ��е�������Cu2+��Cu+����Һ�л����ܴ���������Cl-��
�ʴ�Ϊ��Cu2+��Cu+��Cl-��
��Ϊ����֤��Һ���Ƿ����Cu2+��Cu+����ͨ���������������֤��ȡ�����Ȼ���ͭ���������Թ��У�����Ũ����ʹ���ܽ⣬�ټ����Ȼ�ͭ��Һ���۲�����
�ʴ�Ϊ���Ȼ���ͭ��Ũ����Ȼ�ͭ��
��100mL 0.5mol?L-1 CuCl2��Һ�к�0.05molCuCl2��Cu2++2e-=Cu������0.64gͭת��0.02mol���ӣ�����cʱ��·��һ��ת����0.03mol���ӣ����������Ϣ����ϼ�̬�����ʵ���ɫ�ȵ�һ��̬�����ʵ���ɫ����Ի���0.01mol����Ϊ+2��ͭ���ӵõ�������+1�۵�ͭ���ӣ�Cu2++e-=Cu+�����γɵĵͼ������ӵ����ʵ���Ϊ0.01mol��
�ʴ�Ϊ��0.01��
������������ʵ��̽���⣬��������Լ����Ԫ�ػ�����֪ʶ��Ϊ��Ƶ���㣬������ѧ���ķ��������Ŀ��飬ע�����������ԭ֪ʶ�Լ������غ�ļ��㣬��Ŀ�Ѷ��еȣ�

��ϰ��ϵ�д�
�����Ŀ
�����йظ�����ʴ��������ȷ���ǣ�������
| A��������ˮ�²��ֱ��ڿ�����ˮ���紦������ʴ |
| B����ͭ��ˮ��ͷ���Ӵ��ĸ���ˮ�ܲ�������ʴ |
| C���ɽ��������ֹ������ֱ����Դ�����������Ա��������ܸ�ʴ |
| D���ں����������п�鱣����Dz��ܸ�ʴ�Dz������������������������� |
���и������������¶Ȳ�ͬ���ܷ�����ͬ������ԭ��Ӧ���ǣ�������
| A������ʯ������ |
| B��NaOH��AlCl3��Һ |
| C��Zn��H2SO4��Һ |
| D��Fe��Ũ���� |
�����й�ʵ�������ȷ���ǣ�������
| A����������ƽ��ȡ10.50g�ĸ����NaC1���� |
| B������ʱ�¶ȼ�ˮ����Ӧ������Һ���Ҳ�����ƿ�ײ��Ӵ� |
| C������Ũ�����Ũ����Ļ���ʱ��Ӧ��Ũ���������ӵ�Ũ�����У���ʱ�������ȴ |
| D������Һ��pH����������ˮ��ʪpH��ֽ�����ø���ྻ�IJ�����պ��Һ������ֽ�ϣ��������ɫ������ |
����ʵ����û����ɫ�仯���ǣ�������
| A��������Һ�м���ϡ���� |
| B���������м���Ũ���� |
| C��������Һ�м����� |
| D����ѿ����Һ�����Ƶ�Cu��OH��2��Һ��ϼ��� |
ij��ɫ��Һ��ʹ��ɫʯ����Һ���ɫ����ʵ���ø���Һ�д���Ba2+��NO3-�������Һ�л����ܴ������ڵ��������ǣ�������
| A��NH4+��Mg2+��Cl-��K+ |
| B��Fe2+��Na+��Cl-��I- |
| C��SO42-��HCO3-��Cl-��K+ |
| D��AlO2-��Na+��Cl-��OH- |
����������ȷ���ǣ�������
| A��Ħ�������ĵ�λ�ǣ�g/mol |
| B��ʯӢ�Ļ�ѧʽΪ��CaSiO3 |
| C����ϩ�Ľṹ��ʽΪ��C2H4 |
| D���������Ƶĵ��뷽��ʽΪ��NaOH�TNa++O2-+H+ |
ij��Һ�к��д�����Cl-��SO42-��OH-���������ӣ����ֻȡһ�θ���Һ���ܹ��ֱ�3�����������μ������������ʵ������ʵ�����˳����ȷ���ǣ�������
��1���μ�Mg��NO3��2��Һ��
��2�����ˣ�
��3���μ�AgNO3��Һ��
��4���μ�Ba��NO3��2��Һ��
��1���μ�Mg��NO3��2��Һ��
��2�����ˣ�
��3���μ�AgNO3��Һ��
��4���μ�Ba��NO3��2��Һ��
| A����1����2����4����3����2�� |
| B����4����2����3����2����1�� |
| C����1����2����3����2����4�� |
| D����4����2����1����2����3�� |