��Ŀ����
3��ˮ������EΪ�������ռ������������Ʒ�ɹ˪��E��һ�ֺϳ�·�����£�
��֪��2RCH2CHO$��_{��}^{NaOH��H_{2}O}$

�ش��������⣮
��1��һԪ��A��������������ԼΪ21.6%����A�ķ���ʽΪC4H10O���ṹ������ʾAֻ��һ������A������Ϊ1-������������������F��A��Ϊͬ���칹�壬F�ĺ˴Ź�������ͼ��2��壬�ҷ������Ϊ9��1����F��ŨHBr��Һ���������л���Ľṹ��ʽΪC��CH3��3Br��
��2��B�������Ƶ�Cu��OH��2������Ӧ���÷�Ӧ�Ļ�ѧ����ʽΪCH3CH2CH2CHO+2Cu��OH��2+NaOH$\stackrel{��}{��}$ CH3CH2CH2COONa+Cu2O��+3H2O��
��3��C�����������ŵ�����Ϊ
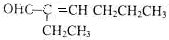 ����Ҫ����C�����������ţ�һ��ȡ�����飬��ʹ�õ��Ⱥ�˳��д�������Լ���������Һ�ȼ���ȩ�����ټ�ϡ����ʹ��Һ�����Ժ���ˮ����̼̼˫����
����Ҫ����C�����������ţ�һ��ȡ�����飬��ʹ�õ��Ⱥ�˳��д�������Լ���������Һ�ȼ���ȩ�����ټ�ϡ����ʹ��Һ�����Ժ���ˮ����̼̼˫������4���ڢ۲��ķ�Ӧ����Ϊ�ӳɷ�Ӧ���ڢܲ��ķ�Ӧ����ΪŨH2SO4�����ȣ�E�Ľṹ��ʽΪ
 ��
��
���� һԪ��A��������������ԼΪ21.6%����ñ���һԪ���Ļ�ѧʽΪCnH2n+2O����Ԫ�ص���������=$\frac{16}{14n+2+16}$��100%=21.6%����n=4����A��ֻ��һ���������Ը�һԪ����1-��������ͭ�����������������£�A��������������B��ȩ����ȩ���������Ƶ�ˮ��Һ������Ӧ���� C����������Ϣ֪��C�Ľṹ��ʽΪ��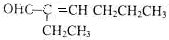 ��C��Ӧ����D��D����Է���������130����C�����������ӳɷ�Ӧ����D����D�Ľṹ��ʽΪ��CH3CH2CH2CH2CH��CH2OH��CH2CH3��D�����ǻ������ᷢ��������Ӧ����E��E�Ľṹ��ʽΪ��
��C��Ӧ����D��D����Է���������130����C�����������ӳɷ�Ӧ����D����D�Ľṹ��ʽΪ��CH3CH2CH2CH2CH��CH2OH��CH2CH3��D�����ǻ������ᷢ��������Ӧ����E��E�Ľṹ��ʽΪ�� ��������ʵĽṹ�����ʽ��н��
��������ʵĽṹ�����ʽ��н��
��� �⣺һԪ��A��������������ԼΪ21.6%����ñ���һԪ���Ļ�ѧʽΪCnH2n+2O����Ԫ�ص���������=$\frac{16}{14n+2+16}$��100%=21.6%����ã�n=4����A��ֻ��һ���������Ը�һԪ����1-��������ͭ�����������������£�A��������������B��ȩ����ȩ���������Ƶ�ˮ��Һ������Ӧ���� C����������Ϣ֪��C�Ľṹ��ʽΪ��CH3CH2CH2CH=C��CH2CH3��CHO��C��Ӧ����D��D����Է���������130����C�����������ӳɷ�Ӧ����D����D�Ľṹ��ʽΪ��CH3CH2CH2CH2CH��CH2OH��CH2CH3��D�����ǻ������ᷢ��������Ӧ����E��E�Ľṹ��ʽΪ�� ��
��
��1��ͨ�����Ϸ���֪��A�ķ���ʽΪ��C4H10O���ṹ������ʾAֻ��һ������A������Ϊ1-������F��A��Ϊͬ���칹�壬��F�������ֲ�ͬ����ԭ�ӣ�������Ϊ9��1����֪FΪ2-��-2-��������F��HBr����ȡ����Ӧ����C��CH3��3Br��
�ʴ�Ϊ��C4H10O��1-������������������C��CH3��3Br��
��2�����������£���ȩ������������ͭ��Ӧ���ɶ����ơ�������ͭ��ˮ����Ӧ����ʽΪ��CH3CH2CH2CHO+2Cu��OH��2+NaOH$\stackrel{��}{��}$ CH3CH2CH2COONa+Cu2O��+3H2O��
�ʴ�Ϊ��CH3CH2CH2CHO+2Cu��OH��2+NaOH$\stackrel{��}{��}$ CH3CH2CH2COONa+Cu2O��+3H2O��
��3��C�Ľṹ��ʽΪ��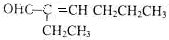 ��C�к���ȩ����̼̼˫�������ܺ���ˮ��Ӧ��Ҫ�������ֹ����ţ���Ӧ����������Һ����ȩ����Ȼ��������ˮ����̼̼˫�������鷽��Ϊ����������Һ�ȼ���ȩ�����ټ�ϡ����ʹ��Һ�����Ժ���ˮ����̼̼˫����
��C�к���ȩ����̼̼˫�������ܺ���ˮ��Ӧ��Ҫ�������ֹ����ţ���Ӧ����������Һ����ȩ����Ȼ��������ˮ����̼̼˫�������鷽��Ϊ����������Һ�ȼ���ȩ�����ټ�ϡ����ʹ��Һ�����Ժ���ˮ����̼̼˫����
�ʴ�Ϊ��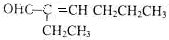 ����������Һ�ȼ���ȩ�����ټ�ϡ����ʹ��Һ�����Ժ���ˮ����̼̼˫����
����������Һ�ȼ���ȩ�����ټ�ϡ����ʹ��Һ�����Ժ���ˮ����̼̼˫����
��4���ڢ۲��ķ�Ӧ��C�����ӳɷ�Ӧ����D����������Ϊ�ӳɷ�Ӧ���ڢܲ��ķ�Ӧ��������Ӧ���������������ķ�Ӧ����֪���÷�Ӧ������Ũ���������������ȣ�ͨ�����Ϸ���֪��E�Ľṹ��ʽΪ�� ��
��
�ʴ�Ϊ���ӳɷ�Ӧ��ŨH2SO4�����ȣ� ��
��
���� ���⿼���л�����ƶϣ���Ŀ�Ѷ��еȣ���ȷ�ƶϳ�A�����ǽⱾ��ؼ�����������Ϣ���з��������ˮ������ȩ����Ϊ�״��㣬ע�����ճ����л���ṹ�����ʣ�

| A�� | ��1 mol Na2CO3����Һ����Na+�ĸ���ΪNA | |
| B�� | ��״���£�1 mol�κ����������Ϊ22.4L | |
| C�� | CH4��Ħ������Ϊ16g | |
| D�� | 3.01��1023��SO2���ӵ�����Ϊ32g |
| A�� | PM2.5��ֱָ��Ϊ2.5pm������ | |
| B�� | PM2.5��ɵ��������������������ܲ�����彡��Σ������ | |
| C�� | �����е�PM2.5����ʾÿ����������ֱ��С�ڻ����2.5um�Ŀ�����ĺ�������PM2.5���ܵ��¿����γ����ܽ� | |
| D�� | �������е�PM2.5���˶�Ӧ���ڲ����˶� |

��ش��������⣮
��1��������������R��ʾ����̼�ڸ����·�Ӧ�Ļ�ѧ����ʽΪ2R+C�T2Fe+2TiO2+CO2�������������Ļ�ѧʽΪFeTiO3������������ŨH2SO4��Ӧ�IJ���֮һ��TiOSO4����Ӧ�����������ɣ��÷�Ӧ�Ļ�ѧ����ʽΪFeTiO3+2H2SO4�TTiOSO4+FeSO4+2H2O
��2���������������м�����м��Ŀ���Ƿ�ֹFe2+��������ʱ��Һ���к���Fe2+��TiO2+������Mg2+�������ӣ������£����Ӧ���������Ksp���±���ʾ��
| �������� | Fe��OH��2 | TiO��OH��2 | Mg��OH��2 |
| Ksp | 8.0��10-16 | 1.0��10-29 | 1.8��10-11 |
����������Fe2+��TiO2+��Mg2+����Һ��ˮϡ�ͣ���������������ɫ�������÷�Ӧ�����ӷ���ʽΪTiO2++2H2O�TTiO��OH��2������H2TiO3��+2H+����
��3���м��Ʒ���뽹̿�������ڸ����·�����Ӧ��ȡTiCl4�ķ���ʽΪTiO2+2C+2Cl2=TiCl4+2CO��Mg��ԭTiCl4�����б�����1070K���¶��½��У�����Ϊ��Ӧ�ÿ��Ƶķ�Ӧ�����Ǹ�����������ȥ���ý������������Ľ���þ���õ��Լ���ϡ���ᣮ
��4����800��1 000��ʱ���TiO2Ҳ���Ƶú����ѣ�װ����ͼ2��ʾ��ͼ��b�ǵ�Դ�������������ĵ缫��ӦʽΪTiO2+4e-�TTi+2O2-��
| A�� | 2Na+2NH3=2NaNH2+H2�� | B�� | NH3+HCl=NH4Cl | ||
| C�� | 4NH3+6NO=5N2+6H2O�� | D�� | 3SiH4+4NH3=Si3N4+12H2 |

��֪��
����ȡTiC14�ķ�Ӧԭ��ΪTiO2��s��+CCl4��g��$\frac{\underline{\;\;��\;\;}}{\;}$TiCl4��g��+CO2��g��
���й����ʵ��������±���
| ���� | �۵�/�� | �е�/�� | ���� |
| CCl4 | -23 | 76 | ��TiCl4���� |
| TiCl4 | -25 | 136 | ����ʪ����������������550��ʱ�ܱ��������� |
��1������N��������Բ����ƿ������A��ʢװ���Լ��Ǽ�ʯ�ң�
��2�����װ�������Եķ����ǹر�ֹˮ��K����װ��E�м���ˮ��û�������¶ˣ���ˮ��M�м�����ˮ��װ��E�ij�
���ܿ������ݲ�������ȥˮ��M��һ��ʱ��������γ�һ��ˮ������˵��װ��������
���ã������������𰸣�
��Ӧǰͨ��N2��Ŀ�����ų�װ���еĿ�������֤��Ӧ��������ˮ�����½���
��3��װ��B����ˮ��������ʹCCl4������
��4��������D�е�Һ̬���������ò���������������
��5��TiC14������TiO2����̿�������ڼ��������·�Ӧ�Ƶã�ͬʱ���������ɣ������ʵ�鷽��̽���������Ƿ�ͬʱ����CO��CO2�������壺��������ͨ������ʯ��ˮ���������ͨ��װ������Cu0�IJ������У�������ʯ��ˮ���
���Ҳ��������к�ɫ�������ɣ���������ͬʱ����CO��C02�������壨������������
������
CO��g��+$\frac{1}{2}$O2��g���TCO2��g����H2=-283.0kJ/mol
��ӦC��s��+O2��g���TCO2��g���ķ�Ӧ��Ϊ��������
| A�� | 172.5 kJ/mol | B�� | -172.5 kJ/mol | C�� | 393.5 kJ/mol | D�� | -393.5 kJ/mol |