��Ŀ����
14���������ӷ���ʽ��д��ȷ���ǣ�������| A�� | Na2SO3��Һ�м���ϡ���SO32-+2H+�TSO2��+H2O | |
| B�� | NH4HSO3��Һ��������NaOH��Һ��ϼ��ȣ�NH4++OH-$\frac{\underline{\;\;��\;\;}}{\;}$NH3��+2H2O | |
| C�� | FeBr2��Һ��ͨ��������Cl2��4Fe2++4Br-+3Cl2�T4Fe3++2Br2+6Cl- | |
| D�� | ��̼�������Һ������������������Һ��Ca2++2HCO3-+2OH-�TCaCO3��+2H2O+CO32- |
���� A��������������ܹ����������������������������ӣ�
B��������������������������������ӷ�Ӧ��
C�������������������ӡ������Ӷ���������
D������������������Ӧ����̼��ơ�̼����غ�ˮ��
��� �⣺A������������Һ�м���ϡ���ᷴӦ�����ӷ���ʽΪ��3SO32-+2NO3-+2H+�T3SO42-+H2O+2NO������A����
B��NH4HSO3��Һ��������NaOH��Һ��ϼ��ȣ����ӷ���ʽ��HSO3-+OH-$\frac{\underline{\;\;��\;\;}}{\;}$SO32-+H2O����B����
C��FeBr2��Һ��ͨ��������Cl2�����ӷ���ʽ��4Fe2++4Br-+3Cl2�T4Fe3++2Br2+6Cl-����C��ȷ��
D����̼�������Һ������������������Һ�����ӷ���ʽ��Ca2++HCO3-+OH-�TCaCO3��+H2O����D����
��ѡ��C��
���� ���⿼�������ӷ���ʽ��д����ȷ��Ӧʵ�ʼ����ӷ���ʽ��д�����ǽ���ؼ���ע�ⷴӦ�������Է�Ӧ��Ӱ�죬ע����������ӵ������ԣ���Ŀ�Ѷ��еȣ�

��ϰ��ϵ�д�
�����Ŀ
17��ij����ѧϰС�����۱�������˵����������ȷ���ǣ�������
�ٵ�ƺ����궼�ǻ���
�����ͺͲ��Ͷ��Ǵ����
�۱��ɱ����Ǵ��������������
�����Ͻ��Ŀǰ��ͨ��Ӳ�Ҷ��ǺϽ�
�������������ǻ����������
������ռ�Ǽ
�߶�����ţ�̶��ǽ��壮
�ٵ�ƺ����궼�ǻ���
�����ͺͲ��Ͷ��Ǵ����
�۱��ɱ����Ǵ��������������
�����Ͻ��Ŀǰ��ͨ��Ӳ�Ҷ��ǺϽ�
�������������ǻ����������
������ռ�Ǽ
�߶�����ţ�̶��ǽ��壮
| A�� | �٢ۢܢ� | B�� | �٢ڢݢ� | C�� | �ۢݢޢ� | D�� | �٢ڢۢ� |
5��TKʱ����2.0L�������ܱ������г���0.10molCOCl2����ӦCOCl2��g��?Cl2��g��+CO��g��������һ��ʱ���ƽ�⣮��Ӧ�����вⶨ�IJ������ݼ��±���
����˵����ȷ���ǣ�������
| t/s | 0 | 2 | 4 | 6 | 8 |
| n��Cl2��/mol | 0 | 0.030 | 0.039 | 0.040 | 0.040 |
| A�� | ���������������䣬�����¶ȣ�ƽ��ʱc��Cl2��=0.022mol•L-1����Ӧ�ġ�H��0 | |
| B�� | ��Ӧ��ǰ2s��ƽ������v��CO��=0.015mol•L-1•S-1 | |
| C�� | ���������������䣬��ʼ�������г���0.12molCOCl2��0.060molCl2��0.060 molCO����Ӧ�ﵽƽ��ǰ�����ʣ�v����v�� | |
| D�� | ���������������䣬��ʼ�������г���0.10molCl2��0.08molCO���ﵽƽ��ʱ��Cl2��ת��С��60% |
2��25��ʱ����������ĵ��볣�����£�
25��ʱ������˵����ȷ���ǣ�������
| ����Ļ�ѧʽ | CH3COOH | HCN | H2S |
| ���볣����25�棩 | 1.8��10-5 | 4.9��10-10 | K1=1.3��10-7 K2=7.1��10-15 |
| A�� | �����ʵ���Ũ�ȵĸ���ҺPH��ϵΪ��PH��Na2S����PH��NaCN����PH��CH3COONa�� | |
| B�� | a mol/LHCN��Һ��b mol/LNaOH��Һ�������ϣ�������Һ��c��Na+����c��CN-������aһ������b | |
| C�� | NaHS��Na2S�Ļ����Һ�У�һ������c��Na+��+c��H+��=c��OH-��+c��HS-��+c��S2-�� | |
| D�� | ijŨ�ȵ�NaCN��Һ��pH=d����������ˮ�������c��H+��=10-dmol/L |
9��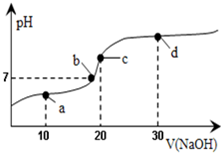 20��ʱ��20mL0.1mol/L������Һ�в��ϵ���0.1mol/LNaOH��aq������ҺpH�仯��ͼ��ʾ���˹�������Һ������Ũ�ȵĹ�ϵ������ǣ�������
20��ʱ��20mL0.1mol/L������Һ�в��ϵ���0.1mol/LNaOH��aq������ҺpH�仯��ͼ��ʾ���˹�������Һ������Ũ�ȵĹ�ϵ������ǣ�������
 20��ʱ��20mL0.1mol/L������Һ�в��ϵ���0.1mol/LNaOH��aq������ҺpH�仯��ͼ��ʾ���˹�������Һ������Ũ�ȵĹ�ϵ������ǣ�������
20��ʱ��20mL0.1mol/L������Һ�в��ϵ���0.1mol/LNaOH��aq������ҺpH�仯��ͼ��ʾ���˹�������Һ������Ũ�ȵĹ�ϵ������ǣ�������| A�� | a�㣺c��CH3COO-����c��Na+����c��H+����c��OH-�� | B�� | b�㣺c��Na+��=c��CH3COO-����c��H+��=c��OH-�� | ||
| C�� | c�㣺c��H+��=c��CH3COOH��+c��OH-�� | D�� | d�㣺c��Na+����c��CH3COO-����c��OH-����c��H+�� |
19�����������������ǽ������ϣ�����Si��Nԭ���������ﵽ8���ӽṹ�����й�������˵������ȷ���ǣ�������
| A�� | ���뵪��ԭ����֮��Ϊ4��3 | |
| B�� | ��ԭ�����ԭ�Ӽ��Թ��ۼ����� | |
| C�� | �۵�ߣ�Ӳ�ȴ�����������ͻ� | |
| D�� | amol�����躬�������������ʵ���Ϊ70amol |
6�� ijͬѧ����Zn��ϡH2SO4�ķ�Ӧ��
ijͬѧ����Zn��ϡH2SO4�ķ�Ӧ��
��1���÷�Ӧ�����ӷ���ʽ��Zn+2H+=Zn2++H2����
��2����H2ʱ����ϡ���������Ũ���ᣬԭ����ŨH2SO4����ǿ�����ԣ���������������
��3����֪��Zn��s��+$\frac{1}{2}$O2��g���TZnO��s����H=-332kJ/mol
ZnO��s��+H2SO4��aq���TZnSO4��aq��+H2O��l����H=-112kJ/mol
H2��g��+$\frac{1}{2}$O2��g���TH2O��l����H=-286kJ/mol
��Zn��ϡH2SO4��Ӧ����1mol H2 ʱ�ķ�Ӧ�ȡ�H=-158kJ/mol��
��4����ͬѧ����ͼװ�ý���ʵ�飬����Ӱ�췴Ӧ���ʵ����أ�ʵ��ʱ���ӶϿ�K��ʼ��ÿ���1���ӣ�����Ͽ���պ�K������������ÿ1 �����ڴ�a��������ˮ�������õ���ˮ���������ʾ��
������Ӧ�����е�ˮ��������ش�
����ˮ����58��34��81��59��˵���ڷ�Ӧ���ڣ��պ�Kʱ�ȶϿ�Kʱ�ķ�Ӧ���ʿ죨��족������������Ҫԭ�����γ�ԭ��ط�Ӧ�ٶȿ죮
����ˮ����102��78��˵���ڷ�Ӧ���ڣ��Ͽ�Kʱ�ķ�Ӧ���ʿ��ڱպ�Kʱ�ķ�Ӧ���ʣ���Ҫԭ���ǶϿ�Kʱ����Һ�е�c��H+�����ڱպ�Kʱ��Һ�е�c��H+����
�۴�����ת����ʽ��ͬ�ĽǶȣ�����ˮ����86��81��117��112����Ҫԭ���ǶϿ�Kʱ����Ӧ�Ļ�ѧ����Ҫת�������ܣ��պ�Kʱ����Ӧ�Ļ�ѧ����Ҫת���ɵ��ܣ�ǰ��ʹ��Һ���¶����ø��ߣ��ʷ�Ӧ���ʸ��죮
 ijͬѧ����Zn��ϡH2SO4�ķ�Ӧ��
ijͬѧ����Zn��ϡH2SO4�ķ�Ӧ����1���÷�Ӧ�����ӷ���ʽ��Zn+2H+=Zn2++H2����
��2����H2ʱ����ϡ���������Ũ���ᣬԭ����ŨH2SO4����ǿ�����ԣ���������������
��3����֪��Zn��s��+$\frac{1}{2}$O2��g���TZnO��s����H=-332kJ/mol
ZnO��s��+H2SO4��aq���TZnSO4��aq��+H2O��l����H=-112kJ/mol
H2��g��+$\frac{1}{2}$O2��g���TH2O��l����H=-286kJ/mol
��Zn��ϡH2SO4��Ӧ����1mol H2 ʱ�ķ�Ӧ�ȡ�H=-158kJ/mol��
��4����ͬѧ����ͼװ�ý���ʵ�飬����Ӱ�췴Ӧ���ʵ����أ�ʵ��ʱ���ӶϿ�K��ʼ��ÿ���1���ӣ�����Ͽ���պ�K������������ÿ1 �����ڴ�a��������ˮ�������õ���ˮ���������ʾ��
| 1����ˮ�������Ͽ�K�� | 34 | 59 | 86 | 117 | �� | 102 |
| 1����ˮ�������պ�K�� | 58 | 81 | 112 | 139 | �� | 78 |
����ˮ����58��34��81��59��˵���ڷ�Ӧ���ڣ��պ�Kʱ�ȶϿ�Kʱ�ķ�Ӧ���ʿ죨��족������������Ҫԭ�����γ�ԭ��ط�Ӧ�ٶȿ죮
����ˮ����102��78��˵���ڷ�Ӧ���ڣ��Ͽ�Kʱ�ķ�Ӧ���ʿ��ڱպ�Kʱ�ķ�Ӧ���ʣ���Ҫԭ���ǶϿ�Kʱ����Һ�е�c��H+�����ڱպ�Kʱ��Һ�е�c��H+����
�۴�����ת����ʽ��ͬ�ĽǶȣ�����ˮ����86��81��117��112����Ҫԭ���ǶϿ�Kʱ����Ӧ�Ļ�ѧ����Ҫת�������ܣ��պ�Kʱ����Ӧ�Ļ�ѧ����Ҫת���ɵ��ܣ�ǰ��ʹ��Һ���¶����ø��ߣ��ʷ�Ӧ���ʸ��죮