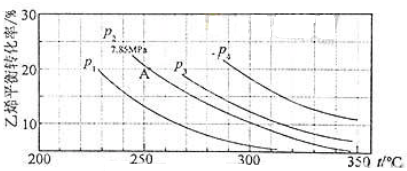
��Ŀ����
X��Y��Z��W��Ԫ�����ڱ���ԭ������������������ֶ�����Ԫ�أ��������Ϣ�����
��1��Wλ��Ԫ�����ڱ��� ���ڵ� �壻W��ԭ�Ӱ뾶��X�� �����С������
��2������Z��Ԫ�ص�ԭ�ӽṹʾ��ͼΪ ��X��WԪ������������Ӧ��ˮ���������ǿ���� �� ���ѧʽ����
��3��Z���Ȼ�����Һ�����Ե�ԭ�������ӷ���ʽ��ʾ�� ��
��4����25�桢101Kpa�£���֪6g��X���嵥����Y2��������ȫȼ�պ�ָ���ԭ״̬������a KJ���÷�Ӧ���Ȼ�ѧ����ʽ�� ��
| Ԫ�� | �� �� �� Ϣ |
| X | X������������Ӧ��ˮ���ﻯѧʽΪH2XO3 |
| Y | Y�ǵؿ��к�����ߵ�Ԫ�� |
| Z | Z�ǵؿ��к�����ߵĽ���Ԫ�� |
| W | W��һ�ֺ��ص�������Ϊ28��������Ϊ14 |
��2������Z��Ԫ�ص�ԭ�ӽṹʾ��ͼΪ
��3��Z���Ȼ�����Һ�����Ե�ԭ�������ӷ���ʽ��ʾ��
��4����25�桢101Kpa�£���֪6g��X���嵥����Y2��������ȫȼ�պ�ָ���ԭ״̬������a KJ���÷�Ӧ���Ȼ�ѧ����ʽ��
���㣺Ԫ�������ɺ�Ԫ�����ڱ����ۺ�Ӧ��
ר�⣺Ԫ����������Ԫ�����ڱ�ר��
������X��Y��Z��W��ԭ������������������ֶ�����Ԫ�أ�Y�ǵؿ��к�����ߵ�Ԫ�أ���YΪOԪ�أ�X������������Ӧ��ˮ���ﻯѧʽΪH2XO3������������ϼ�Ϊ+4����XΪCԪ�أ�Z�ǵؿ��к�����ߵĽ���Ԫ�أ���ZΪAl��W��һ�ֺ��ص�������Ϊ28��������Ϊ14����������=28-14=14����WΪSi���ݴ˽��
���
�⣺X��Y��Z��W��ԭ������������������ֶ�����Ԫ�أ�Y�ǵؿ��к�����ߵ�Ԫ�أ���YΪOԪ�أ�X������������Ӧ��ˮ���ﻯѧʽΪH2XO3������������ϼ�Ϊ+4����XΪCԪ�أ�Z�ǵؿ��к�����ߵĽ���Ԫ�أ���ZΪAl��W��һ�ֺ��ص�������Ϊ28��������Ϊ14����������=28-14=14����WΪSi��
��1��WΪSiԪ�أ�λ��Ԫ�����ڱ��������ڵڢ�A�壻ͬ�������϶���ԭ�Ӱ뾶����ԭ�Ӱ뾶Si��C��
�ʴ�Ϊ��������A����
��2��ZΪAlԪ�أ�ԭ�ӽṹʾ��ͼΪ ���ǽ�����C��Si��������������Ӧ��ˮ���������H2CO3��H2SiO3��
���ǽ�����C��Si��������������Ӧ��ˮ���������H2CO3��H2SiO3��
�ʴ�Ϊ�� ��H2CO3��H2SiO3��
��H2CO3��H2SiO3��
��3��AlCl3��Һ��������ˮ�⣺Al3++3H2O?Al��OH��3+3H+���ƻ�ˮ�ĵ���ƽ�⣬��Һ�����ԣ�
�ʴ�Ϊ��Al3++3H2O?Al��OH��3+3H+��
��4����25�桢101Kpa�£�6g��̼������O2��������ȫȼ�պ�ָ���ԭ״̬������a KJ����1mol̼��ȫȼ�շų�������=a kJ��
=2a kJ���ʸ÷�Ӧ���Ȼ�ѧ����ʽ�ǣ�C��s��+O2��g��=CO2��g����H=-2a kJ/mol��
�ʴ�Ϊ��C��s��+O2��g��=CO2��g����H=-2a kJ/mol��
��1��WΪSiԪ�أ�λ��Ԫ�����ڱ��������ڵڢ�A�壻ͬ�������϶���ԭ�Ӱ뾶����ԭ�Ӱ뾶Si��C��
�ʴ�Ϊ��������A����
��2��ZΪAlԪ�أ�ԭ�ӽṹʾ��ͼΪ
 ���ǽ�����C��Si��������������Ӧ��ˮ���������H2CO3��H2SiO3��
���ǽ�����C��Si��������������Ӧ��ˮ���������H2CO3��H2SiO3���ʴ�Ϊ��
 ��H2CO3��H2SiO3��
��H2CO3��H2SiO3����3��AlCl3��Һ��������ˮ�⣺Al3++3H2O?Al��OH��3+3H+���ƻ�ˮ�ĵ���ƽ�⣬��Һ�����ԣ�
�ʴ�Ϊ��Al3++3H2O?Al��OH��3+3H+��
��4����25�桢101Kpa�£�6g��̼������O2��������ȫȼ�պ�ָ���ԭ״̬������a KJ����1mol̼��ȫȼ�շų�������=a kJ��
| 1mol��12g/mol |
| 6g |
�ʴ�Ϊ��C��s��+O2��g��=CO2��g����H=-2a kJ/mol��
���������⿼��ṹ����λ�ù�ϵӦ�ã��漰��������Ų���Ԫ�������ɡ�����ˮ�⡢�Ȼ�ѧ����ʽ�ȣ���ȷԪ���ǽ���ؼ������ضԻ���֪ʶ�Ĺ��̣��ѶȲ���

��ϰ��ϵ�д�
 ��1����Ԫ�¿�������ĩϵ�д�
��1����Ԫ�¿�������ĩϵ�д�
�����Ŀ
����˵������ȷ���ǣ�������
| A�����ø����������ҩ�ý��һ���˶Խ������Σ�� |
| B��ʳ��һ��������֬�ܴٽ������ijЩά���ص����� |
| C��CH4��Cl2�ڹ��������·�Ӧ�IJ��������������� |
| D��������ϩ�ļӳɣ�������ϩʹ����KMnO4��Һ��ɫ����������ں���̼̼˫���й� |
������������ȷ���ǣ�������
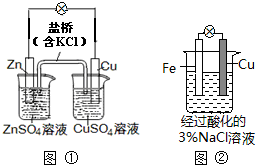

| A��ͼ��������������ҺpH���� |
| B��ͼ���е�����Zn����Cu�������е�Cl-����CuSO4��Һ |
| C��ͼ��������Ӧ��O2+2H2O+4e-�T4OH- |
| D��ͼ���м�������K3[Fe��CN��6]��Һ������ɫ�������� |
����˵������ȷ���ǣ�������
| A����˿����������ø��������ˮ�����ɵõ���-������ |
| B��ijЩ���������γɵķ���ɸ����������״��Ѩ��ͨ������������������ӽ���������������������� |
| C������������۽�һ�����������Ƕ�������ʶ�����Խ���һЩ��ˮ��Һ�н��е���Ӧ������ |
| D������ɨ������������Ӧ��STM������ʵ�ֶ�ԭ�ӻ���ӵIJ��� |
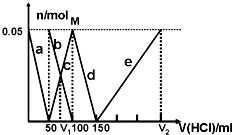 ijNa2CO3��NaAlO2�Ļ����Һ����μ���1mol?L-1�����ᣬ�����Һ�е�CO32-��HCO3-��AlO2-��Al3+���ӵ����ʵ��������������Һ������仯��ϵ��ͼ��ʾ��������˵������ȷ���ǣ�������
ijNa2CO3��NaAlO2�Ļ����Һ����μ���1mol?L-1�����ᣬ�����Һ�е�CO32-��HCO3-��AlO2-��Al3+���ӵ����ʵ��������������Һ������仯��ϵ��ͼ��ʾ��������˵������ȷ���ǣ�������| A��ԭ�����Һ�е�CO32-��AlO2-�����ʵ���֮��Ϊ1��2 |
| B��V1��V2=1��4 |
| C��M��ʱ���ɵ�CO2Ϊ0mol |
| D��a���߱�ʾ�����ӷ���ʽΪ��AlO2-+H++H2O�TAl��OH��3�� |