��Ŀ����
25��ʱ��ȡһ����0.1mol/L�Ĵ����������ʵ�飬��ش����⣺
��1����д�������д��ڵĵ���ƽ�� ��ˮ�ĵ�����⣩��������м������������ƹ��壬��ʱ��Һ��
��ѡ���������С�����䡱����
��2����������м���ϡNaOH��Һ��ǡ���кͣ�д����Ӧ�����ӷ���ʽ�� ��������Һ��pH 7��ѡ��������������T�����������ӷ���ʽ������ԭ�� ��
��3����������м���ϡNaOHֵ��ҺpH=7����ʱc��CH3COO-��=amol?L-����c��Na+��= mol/L��
��4����������м���pH=13��NaOH��Һ���Ҵ�����NaOH��Һ���֮��Ϊ1��1����������Һ�и����ӵ����ʵ���Ũ���ɴ�С��˳���� ��
��1����д�������д��ڵĵ���ƽ��
| c(H+) |
| c(CH3COOH) |
��2����������м���ϡNaOH��Һ��ǡ���кͣ�д����Ӧ�����ӷ���ʽ��
��3����������м���ϡNaOHֵ��ҺpH=7����ʱc��CH3COO-��=amol?L-����c��Na+��=
��4����������м���pH=13��NaOH��Һ���Ҵ�����NaOH��Һ���֮��Ϊ1��1����������Һ�и����ӵ����ʵ���Ũ���ɴ�С��˳����
���㣺�����ʱ�Ķ����жϼ��й�ph�ļ���,���������ˮ��Һ�еĵ���ƽ��
ר�⣺����ƽ������Һ��pHר��
��������1������Ϊ������ʣ�����Һ�в��ֵ������������Ӻ������ӣ������н�������ƾ��壬��Һ�д��������Ũ�����������˴���ĵ��룬�����Ũ������������Ũ�ȼ�С��
��2���������������Ʒ�Ӧ���ɴ����ƺ�ˮ����������Ӳ���ˮ�⣬��Һ��ʾ���ԣ�
��3��ph=7����Һ��ʾ���ԣ�c��H+��=c��OH-��������ݵ���غ�c��Na+��+c��H+��=c��OH-��+c��CH3COO-���жϣ�
��4������������ҺŨ��Ϊ0.1mol/L������Һǡ�÷�Ӧ���ɴ����ƣ���Һ��ʾ���ԣ��ݴ��ж���Һ������Ũ�ȴ�С��
��2���������������Ʒ�Ӧ���ɴ����ƺ�ˮ����������Ӳ���ˮ�⣬��Һ��ʾ���ԣ�
��3��ph=7����Һ��ʾ���ԣ�c��H+��=c��OH-��������ݵ���غ�c��Na+��+c��H+��=c��OH-��+c��CH3COO-���жϣ�
��4������������ҺŨ��Ϊ0.1mol/L������Һǡ�÷�Ӧ���ɴ����ƣ���Һ��ʾ���ԣ��ݴ��ж���Һ������Ũ�ȴ�С��
���
�⣺��1��������Һ�д��ڵ���ƽ��Ϊ��CH3COOH?H++CH3COO-���������������ƾ��壬���������Ũ���������ƴ�����룬ƽ�������ƶ�����Һ��������Ũ�ȼ�С������Ũ��������
�ı�ֵ��С��
�ʴ�Ϊ��CH3COOH?H++CH3COO-����С��
��2��������м���ϡNaOH��Һ��ʹ��ǡ����ȫ��Ӧ�������˴����ƺ�ˮ����Ӧ�����ӷ���ʽΪ��CH3COOH+OH-=CH3COO-+H2O�����ڴ����ˮ�⣺CH3COO-+H2O?CH3COOH+OH-����Һ�ʼ��ԣ���Һ��pH��7��
�ʴ�Ϊ��CH3COOH+OH-=CH3COO-+H2O������CH3COO-+H2O?CH3COOH+OH-��
��3��Һǡ�ó����ԣ���c��H+��=c��OH-�������ݵ���غ�c��Na+��+c��H+��=c��OH-��+c��CH3COO-������c��Na+��=c��CH3COO-��=amol/L��
�ʴ�Ϊ��a��
��4��������pH=13������������Һ��Ũ��Ϊ0.1mol/L������Һ�������1��1��Ϻ�ǡ�÷�Ӧ���ɴ����ƣ����ڴ�������Ӳ���ˮ�⣬��Һ��ʾ���ԣ���c��OH-����c��H+�������ݵ���غ�c��Na+��+c��H+��=c��OH-��+c��CH3COO-���ɵã�c��Na+����c��CH3COO-������Һ������Ũ�ȴ�СΪ��c��Na+����c��CH3COO-����c��OH-����c��H+����
�ʴ�Ϊ��c��Na+����c��CH3COO-����c��OH-����c��H+����
| c(H+) |
| c(CH3COOH) |
�ʴ�Ϊ��CH3COOH?H++CH3COO-����С��
��2��������м���ϡNaOH��Һ��ʹ��ǡ����ȫ��Ӧ�������˴����ƺ�ˮ����Ӧ�����ӷ���ʽΪ��CH3COOH+OH-=CH3COO-+H2O�����ڴ����ˮ�⣺CH3COO-+H2O?CH3COOH+OH-����Һ�ʼ��ԣ���Һ��pH��7��
�ʴ�Ϊ��CH3COOH+OH-=CH3COO-+H2O������CH3COO-+H2O?CH3COOH+OH-��
��3��Һǡ�ó����ԣ���c��H+��=c��OH-�������ݵ���غ�c��Na+��+c��H+��=c��OH-��+c��CH3COO-������c��Na+��=c��CH3COO-��=amol/L��
�ʴ�Ϊ��a��
��4��������pH=13������������Һ��Ũ��Ϊ0.1mol/L������Һ�������1��1��Ϻ�ǡ�÷�Ӧ���ɴ����ƣ����ڴ�������Ӳ���ˮ�⣬��Һ��ʾ���ԣ���c��OH-����c��H+�������ݵ���غ�c��Na+��+c��H+��=c��OH-��+c��CH3COO-���ɵã�c��Na+����c��CH3COO-������Һ������Ũ�ȴ�СΪ��c��Na+����c��CH3COO-����c��OH-����c��H+����
�ʴ�Ϊ��c��Na+����c��CH3COO-����c��OH-����c��H+����
���������⿼������ϵĶ����жϣ���Ŀ�Ѷ��еȣ������漰������ʵĵ��롢�����ˮ������Ũ�ȴ�С�ıȽϵ�֪ʶ�㣬����Ũ�ȴ�С�ıȽ���ѧϰ���ѵ�Ҳ�ǿ��Ե��ȵ㣬���������غ�͵���غ������з������ɣ�ע����ȷ��Һ���������ҺpH�Ĺ�ϵ��

��ϰ��ϵ�д�
 ��У����ϵ�д�
��У����ϵ�д�
�����Ŀ
ij����ѧϰС�����۱�������˵����
�ٴ��κ����궼�ǻ���
�ڴ������ʯ�Ҷ��Ǽ
�۱��ɱ����Ǵ��������ǻ����
�ܲ���ֺ�Ŀǰ��ͨ��Ӳ�Ҷ��ǺϽ�
�������ʳ���ǻ����������
��N2O4�����������������������
�߶����������ǽ��壻
��
C��
C��
C�Dz�ͬ�ֺ��أ�
����˵������ȷ���ǣ�������
�ٴ��κ����궼�ǻ���
�ڴ������ʯ�Ҷ��Ǽ
�۱��ɱ����Ǵ��������ǻ����
�ܲ���ֺ�Ŀǰ��ͨ��Ӳ�Ҷ��ǺϽ�
�������ʳ���ǻ����������
��N2O4�����������������������
�߶����������ǽ��壻
��
12 6 |
13 6 |
14 6 |
����˵������ȷ���ǣ�������
| A���٢ڢۢ� | B���٢ڢߢ� |
| C���ڢۢܢ� | D���ڢݢޢ� |
��ѧ֪ʶ��������������������Ҫ��Ӧ�ã�����˵���в���ȷ���ǣ�������
| A������ʹ��̫���ܡ����ܵ������Դ�ܼ���PM2.5����Ⱦ |
| B����ͭ��ˮ��ͷ���Ӵ��ĸ���ˮ��������ʴ |
| C�����ͷ��к��н϶��NaHCO3����ʹ���Ƴ��ĸ�����ɶ�� |
| D�������ơ�����þ�Ȼ��ý����Ż�ʱ������ʹ����ĭ���������� |
��NA��ʾ����٤������������˵����ȷ���ǣ�������
| A��1 mol�����е���ԭ�ӱ���ԭ����ȫȡ������Ҫ����������Ϊ 4 NA |
| B��1 mol��NO2 ��ˮ��ȫ��Ӧת�Ƶ�����ΪNA |
| C���ڱ�״����2.24 L O2 ��3.2 g O3������ԭ�Ӷ�Ϊ0.2 NA�� |
| D���ڱ�״����2.24 L������������ﺬ���Ӹ���Ϊ0.1 NA�� |
NA��ʾ�����ӵ�����������˵����ȷ���ǣ�������
| A���ڱ�״���£�11.2L�����к���NA����ԭ�� |
| B��2 mol?L-1 ��Na2SO4��Һ�к���4NA��Na+ |
| C��NA��ˮ���ӵ���Է�������֮�͵���ˮ��Ħ������ |
| D�����³�ѹ�£�9��ˮ�����ĵ�����Ϊ5NA |
���ڿ��淴Ӧ2SO2��g��+O2��g��?2SO3��g����H��0�������о�Ŀ�ĺ�ͼʾ������ǣ�������
| A | B | C | D | |
| �о�Ŀ�� | ѹǿ�Է�Ӧ��Ӱ�� | �¶ȶԷ�Ӧ��Ӱ�� | ����O2Ũ�ȶԷ�Ӧ��Ӱ�� | Ũ�ȶ�ƽ�ⳣ����Ӱ�� |
| ͼʾ | 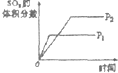 |  | 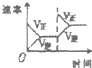 |  |
| A��A | B��B | C��C | D��D |
��NA���������ӵ������������й�������ȷ���ǣ�������
| A��������ΪNA��CO��C2H4����������ԼΪ22.4 L������Ϊ28g |
| B�������£�1L0.1mol��L-1��NH4NO3��Һ��NH4+��NO3-����Ϊ0.2NA |
| C����״���£�4.48 L��ˮ��D20�����е�������Ϊ2NA |
| D��1 mol����-CH3������������Ϊ9NA |

 �л���A��һ����Ҫ�����������м��壬��֪�ṹ��ʽ��ͼ��ʾ��
�л���A��һ����Ҫ�����������м��壬��֪�ṹ��ʽ��ͼ��ʾ��