��Ŀ����
5��������������;�ܹ����ɫ������������ˮ��Һ�׳�˫��ˮ��������������ɱ����Ư�ȣ��Իش��������⣺
��1��д��������������H2O2�����Ȼ����������ӷ�Ӧ����ʽH2O2+2Fe2++2H+�T2Fe3++2H2O��
��2��Na2O2��K2O2�Լ�BaO2���������������ɹ������⣬Ŀǰʵ������ȡ���������ͨ������ij�ֹ�������������ϡ�������ã����˼����Ƶã����������ʺϵĹ���������BaO2��
��3�������[Ca��HCOO��2]�㷺����ʳƷ��ҵ�����ϣ�ʵ������ȡ����Ƶķ���֮һ�ǽ��������ƺͼ�ȩ��Һ���μ�����������Ϊ30%��70%�Ĺ���������Һ�У���÷�Ӧ�Ļ�ѧ����ʽΪCa��OH��2+2HCHO+2H2O2=Ca��HCOO��2+4H2O��
���� ��1��������Һ�й�����������������������Ȼ�����Ϊ�Ȼ������������ⱻ��ԭΪˮ��
��2��������Ŀ��Ϣ��ʵ����ͨ������ij�ֹ�������������ϡ�������ã����˺��Ƶýϴ�����H2O2��Һ��˵�������˳�����
��3�����������ƺͼ�ȩ��Һ���μ�����������Ϊ30%��70%�Ĺ���������Һ�У�����Ca��HCOO��2��H2O��
��� �⣺��1������������H2O2�����Ȼ����������Ȼ�����ˮ����Ӧ�����ӷ���ʽΪ��H2O2+2Fe2++2H+�T2Fe3++2H2O��
�ʴ�Ϊ��H2O2+2Fe2++2H+�T2Fe3++2H2O��
��2��ʵ����ͨ������ij�ֹ�������������ϡ�������ã����˺��Ƶýϴ�����H2O2��Һ��˵�����������ᱵ����������ѡ��BaO2��
�ʴ�Ϊ��BaO2��
��3�����������ƺͼ�ȩ��Һ���μ�����������Ϊ30%��70%�Ĺ���������Һ�У�����Ca��HCOO��2��H2O���䷴Ӧ�ķ���ʽΪ��Ca��OH��2+2HCHO+2H2O2=Ca��HCOO��2+4H2O��
�ʴ�Ϊ��Ca��OH��2+2HCHO+2H2O2=Ca��HCOO��2+4H2O��
���� ���⿼����������ԭ��Ӧ�IJ����жϡ���ѧ��Ӧ����ʽ����д���������ʵ�����Ӧ�ã������ڿ���ѧ���ķ��������ͶԻ���֪ʶ��Ӧ�����������ջ����ǹؼ�����Ŀ�Ѷ��еȣ�

 Сѧ��10���ӿ������100��ϵ�д�
Сѧ��10���ӿ������100��ϵ�д���1��CH3COOH��l��+2O2��g���T2CO2��g��+2H2O��l����H1=-870.3kJ•mol-1
��2��C��s��+O2��g���TCO2��g����H2=-393.5kJ•mol-1
��3��H2��g��+O2��g���TH2O��l����H3=-285.8kJ•mol-1
�����з�Ӧ�ķ�Ӧ��Ϊ��������
2C��s��+2H2��g��+O2��g���TCH3COOH��l��
| A�� | ��H=+488.3 kJ•mol-1 | B�� | ��H=-244.15 kJ•mol-1 | ||
| C�� | ��H=-977.6 kJ•mol-1 | D�� | ��H=-488.3 kJ•mol-1 |
| A�� | �÷�Ӧ��X����ΪO2 | |
| B�� | ��Ӧ��Na2O2ֻ�������� | |
| C�� | �÷�Ӧ�з���������Ӧ�Ĺ���ֻ��FeSO4��Na2FeO4 | |
| D�� | ÿ����lmol Na2FeO4����Ӧ������ת��4 mol e- |
| A�� | �������� | B�� | �����ס��� | C�� | ͭ�������� | D�� | п���ӡ��� |
| A�� | CH3CH2Br+NaOH$��_{��}^{H_{2}O}$CH2=CH2��+NaBr+H2O | |
| B�� |  +3Br2�� +3Br2�� | |
| C�� | nCH2=CH-CH3$\stackrel{һ������}{��}$ | |
| D�� | CH3CHO+NaOH+2Cu��OH��2$\stackrel{����}{��}$ CH3COONa+Cu2O��+3H2O�� |
 �����������ѧ��Fulvio Cacace���˻���˼��������о��������̬N4���ӣ�����ӽṹ��ͼ��ʾ����֪����1mol N-N����167kJ����������1mol N��N�ų�942kJ����������������Ϣ�����ݣ��ж�����˵����ȷ���ǣ�������
�����������ѧ��Fulvio Cacace���˻���˼��������о��������̬N4���ӣ�����ӽṹ��ͼ��ʾ����֪����1mol N-N����167kJ����������1mol N��N�ų�942kJ����������������Ϣ�����ݣ��ж�����˵����ȷ���ǣ�������| A�� | N4����һ�����͵Ļ����� | |
| B�� | N4������N-N����Ϊ109��28�� | |
| C�� | N4�����д��ڷǼ��Լ� | |
| D�� | 1 mol N4ת���N2������882 kJ���� |
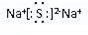 ��
�� ʵ����Ϊ��֤±�ص��������Ե�ǿ����ijС������ͼ��ʾװ�ý���ʵ�飨�г���������ȥ���������Ѽ��飩��
ʵ����Ϊ��֤±�ص��������Ե�ǿ����ijС������ͼ��ʾװ�ý���ʵ�飨�г���������ȥ���������Ѽ��飩��