��Ŀ����
8����1��5.4gˮ��29.4g���������ķ�������ȣ�����������ԭ����֮����1��4����ԭ����֮����1��1����2��12.4g Na2X����0.4mol Na+��Na2X��Ħ������Ϊ62g/mol������Է�������Ϊ62��X�����ԭ������Ϊ16�������ʵĻ�ѧʽΪNa2O��
��3����ͬ״���£�һ���������̬�⻯��H2X�������ǵ����NH3��2������X�����ԭ������Ϊ32��
��4����״����ij����A���ܶ�Ϊ1.518g/L����A��Ħ������Ϊ34g/mol��
��5���ٱ�״���£�22.4L CH4����1.5mol NH3����1.806��1024��H2O���ܱ�״���£�73g HCl���������������к�Hԭ�Ӹ����ɶൽ�ٵ�˳���Ǣۣ��ڣ��٣��ܣ�
���� ��1������n=$\frac{m}{M}$����ˮ�����ʵ�����ˮ�����Ậ�з�����Ŀ��ȣ���������ʵ�����ȣ��ٸ���m=nM����������������Ϸ���ʽ������ԭ����Ŀ֮�ȡ���ԭ����Ŀ֮�ȣ�
��2���������������ʵ�������Na2X�����ʵ������ٸ���M=$\frac{m}{n}$����Na2X��Ħ����������������X�����ԭ������������ȷ��X��Ԫ�ط�����д��ѧʽ��
��3����ͬ�����£������ܶ�֮�ȵ�������Է�������֮�ȣ��ݴ˼���H2X��Է�����������������X���ԭ��������
��4������M=��Vm���㣻
��5������n=$\frac{V}{{V}_{m}}$=$\frac{m}{M}$=$\frac{N}{{N}_{A}}$������顢ˮ��HCl���ʵ������ٽ�Ϸ���ʽ����Hԭ�����ʵ�����
��� �⣺��1��ˮ�����ʵ���Ϊ$\frac{5.4g}{18g/mol}$=0.3mol��ˮ�����Ậ�з�����Ŀ��ȣ���������ʵ�����ȣ�����������Ϊ0.3mol��98g/mol=29.4g��������ԭ����Ŀ֮��Ϊ1��4��������ԭ����Ŀ֮��Ϊ2��2=1��1��
�ʴ�Ϊ��29.4��1��4��1��1��
��2��12.4g Na2X�����ʵ���Ϊ$\frac{0.4mol}{2}$=0.2mol��Na2X��Ħ������Ϊ$\frac{12.4g}{0.2mol}$=62g/mol��Na2X����Է�������Ϊ62g��X�����ԭ����������=62-23��2=16��ΪXΪ��Ԫ�أ������ʻ�ѧʽΪNa2O��
�ʴ�Ϊ��62g/mol��62��16��Na2O��
��3����ͬ״���£�һ���������̬�⻯��H2X�������ǵ����NH3��2������H2X���ܶ���NH3��2������ͬ�����£������ܶ�֮�ȵ�������Է�������֮�ȣ���H2X����Է�������Ϊ17��2=34����X���ԭ������Ϊ34-2=32��
�ʴ�Ϊ��32��
��4����״����ij����A���ܶ�Ϊ1.518g/L����A��Ħ������Ϊ1.518g/L��22.4L/mol=34g/mol��
�ʴ�Ϊ��34g/mol��
��5���ٱ�״���£�22.4L CH4Ϊ1mol������Hԭ��Ϊ1mol��4=4mol��
��1.5mol NH3����Hԭ��Ϊ1.5mol��3=4.5mol��
��1.806��1024��H2O���ʵ���Ϊ$\frac{1.806��1{0}^{24}}{6.02��1{0}^{23}mo{l}^{-1}}$=3mol������Hԭ��Ϊ3mol��2=6mol��
�ܱ�״���£�73g HCl�����ʵ���Ϊ$\frac{73g}{36.5g/mol}$=2mol������Hԭ��Ϊ2mol��
���������к�Hԭ�Ӹ����ɶൽ�ٵ�˳���ǣ��ۣ��ڣ��٣��ܣ�
�ʴ�Ϊ���ۣ��ڣ��٣��ܣ�
���� ���⿼�����ʵ����йؼ��㣬�ѶȲ������������ʵ���Ϊ���ĵ��йؼ��㣬�����ڻ���֪ʶ�Ĺ��̣�

 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�| A�� | �ڿ��淴Ӧ�У�����Ӧ�Ļ�ѧ��Ӧ��������ֵ���淴Ӧ�Ļ�ѧ��Ӧ�����Ǹ�ֵ | |
| B�� | ��ѧ��Ӧ����Ϊ0.8 mol•L-1•s-1��ָ1����ʱij���ʵ�Ũ��Ϊ0.8 mol•L-1 | |
| C�� | ���ݻ�ѧ��Ӧ���ʵĴ�С����֪����ѧ��Ӧ���еĿ��� | |
| D�� | ��ѧ��Ӧ�����ʿ�����g•��L•s��-1��Ҳ������kg•��L•s��-1������������t•��L•s��-1��ʾ |
| A�� | �� | B�� | þ | C�� | �� | D�� | ͭ |
| A�� | v��O2��=0.001mol/��L•S�� | B�� | v��NH3��=0.002 mol/��L•S�� | ||
| C�� | v��H2O��=0.003 mol/��L•h�� | D�� | v��NO��=0.008 mol/��L•S�� |
| A�� | Na+��Al3+��Cl-��CO32- | B�� | Fe2+��H+��SO42-��NO3- | ||
| C�� | K+��Fe3+��NO3-��SCN- | D�� | Mg2+��NH4+��Cl-��SO42- |
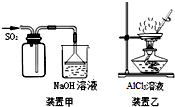
| A�� | ��װ�ü��ռ�SO2 | |
| B�� | ��װ�����Ʊ�AlCl3���� | |
| C�� | �к͵ζ�ʱ����ƿ�ô�װҺ��ϴ | |
| D�� | ʹ�÷�Һ©��������ƿʱ����Ҫ����Ƿ�©Һ |
| A�� | 1��2��3 | B�� | 1��3��2 | C�� | 2��3��1 | D�� | 3��2��1 |
 ���������仯����ķ���ͼ����ش��������⣺
���������仯����ķ���ͼ����ش��������⣺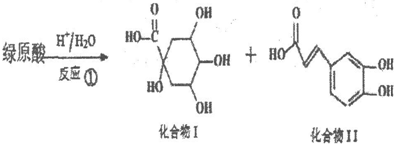
 ��
��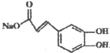 �������Լ�X��NaHCO3��Һ��
�������Լ�X��NaHCO3��Һ��