��Ŀ����
 X��Y��Z��W��T�Ƕ����ڱ��е�5������Ԫ�أ�X��̬ԭ�Ӻ���L��p����������s��������Y��һ�ֺ��ص�������Ϊ19������������������1��T-���Ӻ�����������ԭ�ӵĺ����������ͬ��X��Y��Z��W�ĵ�һ�����������ǵĺ˵�����Ĺ�ϵ��ͼ��ʾ��
X��Y��Z��W��T�Ƕ����ڱ��е�5������Ԫ�أ�X��̬ԭ�Ӻ���L��p����������s��������Y��һ�ֺ��ص�������Ϊ19������������������1��T-���Ӻ�����������ԭ�ӵĺ����������ͬ��X��Y��Z��W�ĵ�һ�����������ǵĺ˵�����Ĺ�ϵ��ͼ��ʾ����1��Xλ��Ԫ�����ڱ���
��2��Y��T�γɵ���̬�⻯���н��ȶ�����
��3��XY4��������������
��4��Z�Ļ�̬ԭ����Χ���ӣ��۵��ӣ��Ų�ʽΪ
���㣺λ�ýṹ���ʵ����ϵӦ��
ר�⣺Ԫ����������Ԫ�����ڱ�ר��
������X��̬ԭ�Ӻ���L��p����������s����������XΪCԪ�أ�Y��һ�ֺ��ص�������Ϊ19������������������1����YΪFԪ�أ�T-���Ӻ�����������ԭ�ӵĺ����������ͬ����TΪClԪ�أ�W�ĵ�һ�����ܸ�Z��W��Z�ĵ�һ�����ܽ���X��Y֮�䣬����WΪNԪ�أ�ZΪOԪ�أ��ݴ˴��⣻
���
�⣺X��̬ԭ�Ӻ���L��p����������s����������XΪCԪ�أ�Y��һ�ֺ��ص�������Ϊ19������������������1����YΪFԪ�أ�T-���Ӻ�����������ԭ�ӵĺ����������ͬ����TΪClԪ�أ�W�ĵ�һ�����ܸ�Z��W��Z�ĵ�һ�����ܽ���X��Y֮�䣬����WΪNԪ�أ�ZΪOԪ�أ�
��1��XΪCԪ�أ�λ�����ڱ��еڶ����ڵڢ�A�壬����N��ԭ�Ӱ뾶С��C������N-H�ļ��ܴ���C-H������CN��2��ÿ��ԭ�ӵ��������Ӷ�����8�����ȶ��ṹ�������ʽΪ ��
��
�ʴ�Ϊ��������A��N-H�� ��
��
��2��Y��T�γɵ���̬�⻯��ֱ�ΪHF��HCl������Ԫ�������ɣ�ͬ����Ԫ�ش��ϵ����⻯����ȶ�������������HF��HCl�ȶ�������HF����֮�������������HF�ķе�ϸߣ��ʴ�Ϊ��HF��HF��
��3��XY4ΪCF4���Ƿ��Ӿ��壬�ʴ�Ϊ�����ӣ�
��4��ZΪOԪ�أ�ԭ������Ϊ8���۵�����Ϊ6���������Ļ�̬ԭ����Χ���ӣ��۵��ӣ��Ų�ʽΪ2s22p4���ʴ�Ϊ��2s22p4��
��1��XΪCԪ�أ�λ�����ڱ��еڶ����ڵڢ�A�壬����N��ԭ�Ӱ뾶С��C������N-H�ļ��ܴ���C-H������CN��2��ÿ��ԭ�ӵ��������Ӷ�����8�����ȶ��ṹ�������ʽΪ
 ��
���ʴ�Ϊ��������A��N-H��
 ��
����2��Y��T�γɵ���̬�⻯��ֱ�ΪHF��HCl������Ԫ�������ɣ�ͬ����Ԫ�ش��ϵ����⻯����ȶ�������������HF��HCl�ȶ�������HF����֮�������������HF�ķе�ϸߣ��ʴ�Ϊ��HF��HF��
��3��XY4ΪCF4���Ƿ��Ӿ��壬�ʴ�Ϊ�����ӣ�
��4��ZΪOԪ�أ�ԭ������Ϊ8���۵�����Ϊ6���������Ļ�̬ԭ����Χ���ӣ��۵��ӣ��Ų�ʽΪ2s22p4���ʴ�Ϊ��2s22p4��
������������Ҫ������Ԫ�����ڱ���Ԫ�������ɡ���������Ų����й�֪ʶ���ѶȲ�����ʱע�����֪ʶ��������ã�

��ϰ��ϵ�д�
�����Ŀ
�������ӷ���ʽ������ǣ�������
| A����Mg��HCO3��2��Һ�м�������� NaOH��Һ��Mg2++2HCO3-+4OH-=Mg��OH��2��+2CO32-+2H2O |
| B���������ᱵ�����м���ϡ���3BaSO3+2H++2NO3-=3BaSO4��+2NO��+H2O |
| C����������Һ�еμӹ���ϡ���[Ag��NH3��2]++2H+=Ag++2NH4+ |
| D����NH4HSO4ϡ��Һ����μ���Ba��OH��2ϡ��Һ��SO42-�պó�����ȫ��Ba2++2OH-+NH4++H++SO42-=BaSO4��+NH3?H2O+H2O |
ij��Һ�п��ܺ�������6�������еļ��֣�NH4+��A13+��Mg2+��CO32-��Cl-��SO42-��Ϊȷ����Һ����ɣ���ȡ100mL�ֳ����ȷ���Һ��������ʵ�飺
��1�����һ����Һ�м��� AgNO3��Һ�г���������
��2����ڶ�����Һ�м�������NaOH��Һ��ַ�Ӧ�����յõ�����0.58g��ͬʱ�ռ�������0.03mol��������ȫ������Һ���ݳ�����
��3�����������Һ�м�������BaCl2��Һ�������ữ����ַ�Ӧ�õ�����6.99g��
�ɴ˿�֪�����й���ԭ��Һ��ɵ���ȷ�����ǣ�������
��1�����һ����Һ�м��� AgNO3��Һ�г���������
��2����ڶ�����Һ�м�������NaOH��Һ��ַ�Ӧ�����յõ�����0.58g��ͬʱ�ռ�������0.03mol��������ȫ������Һ���ݳ�����
��3�����������Һ�м�������BaCl2��Һ�������ữ����ַ�Ӧ�õ�����6.99g��
�ɴ˿�֪�����й���ԭ��Һ��ɵ���ȷ�����ǣ�������
| A����Һ��SO42-��Ũ����0.3 mol/L |
| B����Һ��һ������A13+��NH4+ |
| C��һ��������Mg2+�����ܴ���A13+ |
| D��һ������Cl- ���ܺ�CO32- |
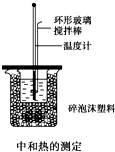 50mL 0.50mol/L������50mL 0.55mol/L NaOH��Һ����ͼ��ʾ��װ���н����кͷ�Ӧ��ͨ���ⶨ��Ӧ���������ų��������ɼ����к��ȣ��ش��������⣺
50mL 0.50mol/L������50mL 0.55mol/L NaOH��Һ����ͼ��ʾ��װ���н����кͷ�Ӧ��ͨ���ⶨ��Ӧ���������ų��������ɼ����к��ȣ��ش��������⣺ ��ͼ��ѧУʵ���Ҵӻ�ѧ�Լ��̵���ص������Լ���ǩ�ϵIJ������ݣ��ݴˣ��ش����⣺
��ͼ��ѧУʵ���Ҵӻ�ѧ�Լ��̵���ص������Լ���ǩ�ϵIJ������ݣ��ݴˣ��ش����⣺