��Ŀ����
��ѧʵ��С����ʵ��������500mL 0.1mol?L-1 Na2CO3��Һ��ʵ�����˳�����£�

��1��ͼ���������Na2CO3���������Ϊ ����ʵ��ʱ�������Ⱥ�˳��Ϊ �����ţ���
��2������ƿʹ��ǰӦ ����ѧ��ѧʵ�鳣��������ʹ��ǰ������ƿʹ������ͬ�����IJ�ͬ�ಣ���������� �� ��
��3��������Na2CO3��Һʱ�����в���ʹ������ҺŨ��ƫ�͵��� ��
A��ԭ����ƿϴ����δ���
B������Na2CO3��Һʱ��δ��ȴ�����¾�ת������ƿ���ƣ�
C������ʱҺ�泬���˿̶��ߣ��ý�ͷ�ι���������Һ�����̶��ߣ�
D������Na2CO3����ʱ��ҩƷ������Ŵ���
��ʵ�������ý������Ʊ���������������������3�ַ�����
����a��Al
Al3+
Al��OH��3��
����b��Al
AlO
Al��OH��3��
����c��
��Al��OH��3
��4���Ʊ���ͬ���ʵ�������������������ͼ����ٵ��Ƿ��� ���������һ����Ӧ�����ӷ���ʽΪ ��

��1��ͼ���������Na2CO3���������Ϊ
��2������ƿʹ��ǰӦ
��3��������Na2CO3��Һʱ�����в���ʹ������ҺŨ��ƫ�͵���
A��ԭ����ƿϴ����δ���
B������Na2CO3��Һʱ��δ��ȴ�����¾�ת������ƿ���ƣ�
C������ʱҺ�泬���˿̶��ߣ��ý�ͷ�ι���������Һ�����̶��ߣ�
D������Na2CO3����ʱ��ҩƷ������Ŵ���
��ʵ�������ý������Ʊ���������������������3�ַ�����
����a��Al
| H2SO4 |
| NaOH |
����b��Al
| NaOH |
| H2SO4 |
����c��
|
��4���Ʊ���ͬ���ʵ�������������������ͼ����ٵ��Ƿ���
���㣺����һ�����ʵ���Ũ�ȵ���Һ
ר�⣺ʵ����
��������1������n=C?V��m=n?M�����㣻Ȼ���������һ�����ʵ���Ũ�ȵIJ����ǣ��������ܽ⣬��Һ��ϴ�ӣ����ݣ�ҡ�ȣ�װƿ�����ǣ�
��2����ƿ���ͻ���������ʹ��ǰ����Ҫ��©���ݴ˷�����
��3������c=
��ͨ������n��V�ı仯������c�ı仯��
��4�����ݽ����������Լ��ͼ����Ӧ�Ļ�ѧ����ʽ������ش𣬸������ӷ���ʽ����д�������������
��2����ƿ���ͻ���������ʹ��ǰ����Ҫ��©���ݴ˷�����
��3������c=
| n |
| V |
��4�����ݽ����������Լ��ͼ����Ӧ�Ļ�ѧ����ʽ������ش𣬸������ӷ���ʽ����д�������������
���
�⣺��1������n=C?V��֪n=0.5L��0.1mol?L-1=0.05mol������m=n?M=0.05mol��106g/mol=5.3g��
����һ�����ʵ���Ũ�ȵIJ����ǣ��������ܽ⣬��Һ��ϴ�ӣ����ݣ�ҡ�ȣ�װƿ������ȷ��˳���ǣ��ڢܢޢݢ٢ۣ�
�ʴ�Ϊ��5.3g���ڢܢޢݢ٢ۣ�
��2��ʹ������ƿ�ĵ�һ�������Ǽ������ƿ�Ƿ�©ˮ������Ҫ��©�Ļ��з�Һ©���͵ζ��ܣ��ʴ�Ϊ����©����Һ©�����ζ��ܺͣ�
��3��A��ԭ����ƿϴ����δ�����������ҺŨ����Ӱ�죬��A��ѡ��
B������Na2CO3��Һʱ��δ��ȴ�����¾�ת������ƿ���ƣ��ᵼ����Һ���ƫС����Ũ��ƫ�ߣ���B��ѡ��
C������ʱҺ�泬���˿̶��ߣ��ý�ͷ�ι���������Һ�����̶��ߣ���ҺŨ��ƫ�ͣ���Cѡ��
D������Na2CO3����ʱ��ҩƷ������Ŵ��̣����³�����ҩƷ������ƫС����������ҺŨ��ƫ�ͣ���Dѡ��
��ѡCD��
��4�����ݽ����������Լ��ͼ����Ӧ�Ļ�ѧ����ʽ���Եó���Al��3H+��Al3+��Al��OH��3��
Al��OH-��[Al��OH��4]-��Al3++3[Al��OH��4]-=4Al��OH��3���������Ʊ���ͬ���ʵ�������������������ͼ����ٵ��Ƿ���C��
���һ����Ӧ�Ļ�ѧ����ʽΪ��Al2��SO4��3+6NaAlO2+6H2O=8Al��OH��3��+3Na2SO4��������д���ӷ���ʽʱ�ܲ����ǿ�ᡢǿ��������ο�֪��Al2��SO4��3��NaAlO2��Na2SO4���Բ𣬹����ӷ���ʽΪ��Al3++3AlO2-+6H2O=4Al��OH��3�����ʴ�Ϊ��C��Al3++3AlO2-+6H2O=4Al��OH��3����
����һ�����ʵ���Ũ�ȵIJ����ǣ��������ܽ⣬��Һ��ϴ�ӣ����ݣ�ҡ�ȣ�װƿ������ȷ��˳���ǣ��ڢܢޢݢ٢ۣ�
�ʴ�Ϊ��5.3g���ڢܢޢݢ٢ۣ�
��2��ʹ������ƿ�ĵ�һ�������Ǽ������ƿ�Ƿ�©ˮ������Ҫ��©�Ļ��з�Һ©���͵ζ��ܣ��ʴ�Ϊ����©����Һ©�����ζ��ܺͣ�
��3��A��ԭ����ƿϴ����δ�����������ҺŨ����Ӱ�죬��A��ѡ��
B������Na2CO3��Һʱ��δ��ȴ�����¾�ת������ƿ���ƣ��ᵼ����Һ���ƫС����Ũ��ƫ�ߣ���B��ѡ��
C������ʱҺ�泬���˿̶��ߣ��ý�ͷ�ι���������Һ�����̶��ߣ���ҺŨ��ƫ�ͣ���Cѡ��
D������Na2CO3����ʱ��ҩƷ������Ŵ��̣����³�����ҩƷ������ƫС����������ҺŨ��ƫ�ͣ���Dѡ��
��ѡCD��
��4�����ݽ����������Լ��ͼ����Ӧ�Ļ�ѧ����ʽ���Եó���Al��3H+��Al3+��Al��OH��3��
Al��OH-��[Al��OH��4]-��Al3++3[Al��OH��4]-=4Al��OH��3���������Ʊ���ͬ���ʵ�������������������ͼ����ٵ��Ƿ���C��
���һ����Ӧ�Ļ�ѧ����ʽΪ��Al2��SO4��3+6NaAlO2+6H2O=8Al��OH��3��+3Na2SO4��������д���ӷ���ʽʱ�ܲ����ǿ�ᡢǿ��������ο�֪��Al2��SO4��3��NaAlO2��Na2SO4���Բ𣬹����ӷ���ʽΪ��Al3++3AlO2-+6H2O=4Al��OH��3�����ʴ�Ϊ��C��Al3++3AlO2-+6H2O=4Al��OH��3����
���������⿼��ѧ��һ�����ʵ���Ũ����Һ�����ƺ�ҵ��ý�������ʵ�鷽��֪ʶ����һ����ҵ�����ͻ�ѧ��ϵ���Ŀ���ѶȽϴ�

��ϰ��ϵ�д�
�����Ŀ
��0.1mol�������ʷֱ����1000mL����ˮ�У��ָ������£�������Һ��������Ũ�ȵĴ�С˳���ǣ�����������Һ����仯���Բ��ƣ���Na2O ��Na2O2 ��NaOH ��Na��
| A���٣��ڣ��ۣ��� |
| B���٣��ڣ��ܣ��� |
| C����=�ڣ���=�� |
| D����=�ڣ��ۣ��� |
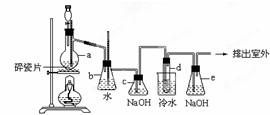 1��2-��������������Ϳ����������Ӽ���������������ɫҺ�壬�ܶ���2.18��/����3���е�131.4�棬�۵�9.79�棬������ˮ�������ڴ����ѡ���ͪ���л��ܼ�����ʵ���п�������ͼ��ʾװ���Ʊ�1��2-�������飮���з�Һ©������ƿa��װ���Ҵ���Ũ����Ļ��Һ���Թ�d��װ��Ũ�壨���渲������ˮ��������д���пհף�
1��2-��������������Ϳ����������Ӽ���������������ɫҺ�壬�ܶ���2.18��/����3���е�131.4�棬�۵�9.79�棬������ˮ�������ڴ����ѡ���ͪ���л��ܼ�����ʵ���п�������ͼ��ʾװ���Ʊ�1��2-�������飮���з�Һ©������ƿa��װ���Ҵ���Ũ����Ļ��Һ���Թ�d��װ��Ũ�壨���渲������ˮ��������д���пհף�
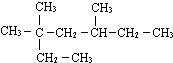
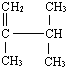
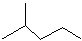 ��ʾ�ķ���ʽ
��ʾ�ķ���ʽ