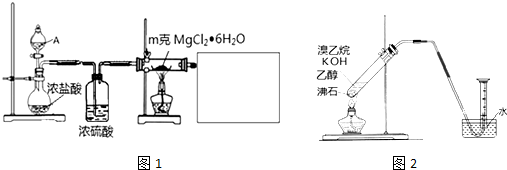
��Ŀ����
6��С���������о��¶ȶԷ�Ӧ���ʵ�Ӱ�족ʵ��ʱ����ȡ����ֻ�Թܣ�������4mL 0.01mol/L��KMnO4������Һ��2mL 0.1mol/L H2C2O4���Ҷ��ᣬ�������ᣬ�Ƕ�Ԫ���ᣩ��Һ����A�Թ�������ˮ�У�B�Թ�������ˮ�У���¼��Һ��ɫ�����ʱ�䣮����Ҫ���������ữKMnO4��Һ������ĸ��A������ B������ C������
��ɫ����ʱ��tA��tB�����������=����������
��д���÷�Ӧ�����ӷ���ʽ5H2C2O4+2MnO4-+6H+=10CO2��+2Mn2++8H2O��
��2��ʵ������ƿ������ɳ���Ҷ�����Ʒ��С�����������Ӧ��ԭ�����ⶨ�京�����������Ϊ��
������250mL��Һ��ȷ����5.0g�Ҷ�����Ʒ�����250mL��Һ��
�ڵζ���ȷ��ȡ25.00mL������Һ����ƿ�У����������ữ����0.1000mol/LKMnO4��Һװ����ʽ�����ʽ����ʽ�����ζ��ܣ����еζ�������
����Ӧ�����ɵ������Ӿ��д����ã����������ɫ��ӿ죬���������һ�θ��������Һ����ƿ�ڵ���ɫǡ�ñ���Ϻ�ɫ�Ұ���Ӳ��仯��֤���ﵽ�ζ��յ㣮
�ۼ��㣺���ظ���������2�Σ���¼ʵ���������£�
| ��� | �ζ�ǰ������mL�� | �ζ��������mL�� |
| 1 | 0.00 | 20.10 |
| 2 | 1.00 | 20.90 |
| 3 | 0.00 | 21.10 |
�������������в����ᵼ�²ⶨ���ƫ�ߵ���ACD��
A��δ�ñ�Ũ�ȵ�����KMnO4��Һ��ϴ�ζ���
B���ζ�ǰ��ƿ������ˮ
C���ζ�ǰ�ζ��ܼ��첿�������ݣ��ζ���������ʧ
D����С�Ľ���������KMnO4��Һ������ƿ��
E���۲����ʱ���ζ�ǰ���ӣ��ζ����ӣ�
���� ��1���ٸ�����ؾ���ǿ�����ԣ�Ҫ�ữ���������Һ��Ҫѡ����ԭ�Ե��ᣬ�¶�Խ�߷�Ӧ����Խ�죬����ɫʱ��Խ�̣�
�����������£�������������������������ɶ�����̼����������ԭ���������ӣ�ͬʱ����ˮ��
��2����������Һֻ��ʢ������ʽ�ζ����У��������д����ö����·�Ӧ���ʼӿ죬���������һ�θ��������Һ����ƿ�ڵ���ɫǡ�ñ���Ϻ�ɫ�Ұ���Ӳ��仯��֤���ﵽ�յ㣻
��ƽ��ֵ�������ĸ�����ص������������ı�ֵ�����ݸ�����غͲ���֮��Ĺ�ϵʽ���㣻
�ܸ���C=$\frac{n}{V}$�жϲ���������n��V��Ӱ���жϣ�
��� �⣺��1���ٸ�����ؾ���ǿ�����ԣ�Ҫ�ữ���������Һ��Ҫѡ����ԭ�Ե��ᣬһ��ѡϡ���ᣬ�¶�Խ�߷�Ӧ����Խ�죬����ɫʱ��Խ�̣�������ɫ����ʱ��tA��tB���ʴ�Ϊ�����ᣬ����
�����������£�������������������������ɶ�����̼����������ԭ���������ӣ�ͬʱ����ˮ�����������ӷ�Ӧ����ʽΪ��5H2C2O4+2MnO4-+6H+=10CO2��+2Mn2++8H2O��
�ʴ�Ϊ��5H2C2O4+2MnO4-+6H+=10CO2��+2Mn2++8H2O��
��2����������Һֻ��ʢ������ʽ�ζ����У��������Ը��������ҺӦ��ʢ������ʽ�ζ����У�����������ӱ���ԭ���ɵ��������д����ö����·�Ӧ���ʼӿ죬���������һ�θ��������Һ����ƿ�ڵ���ɫǡ�ñ���Ϻ�ɫ�Ұ���Ӳ��仯��֤���ﵽ�յ㣬
�ʴ�Ϊ����ʽ����Ӧ�����ɵ������Ӿ��д����ã����������ɫ��ӿ죬���������һ�θ��������Һ����ƿ�ڵ���ɫǡ�ñ���Ϻ�ɫ�Ұ���Ӳ��仯��
�۵���������������һ�κ͵ڶ������ϴ�����Ҫ��ȥ��������KMnO4��Һ��ƽ�����=$\frac{��20.10-0.00��+��20.90-1.00��}{2}$mL=20.00mL��
����Ʒ�Ĵ���Ϊx��
5H2C2O4+2MnO4-+6H+=10CO2��+2Mn2++8H2O
450g 2mol
5.0x��$\frac{1}{10}$g ��0.1��0.020��mol
x=$\frac{0.1��0.020��450}{2��5.0��0.1}$=90.00%��
�ʴ�Ϊ��90.00%��
��A����ʽ�ζ���ˮϴ��δ�ô���Һ��ϴ���ᵼ�����Ը������Ũ��ƫС����Ҫ����������ƫ�ⶨֵƫ����ȷ��
B����ƿ����ˮ����ʵ����Ӱ�죬�ʴ���
C�����ܼ��첿�������ݣ��ζ�����ʧ���ᵼ�²ⶨ����������ƫ�ⶨֵƫ����ȷ��
D����С�Ľ���������KMnO4��Һ������ƿ�⣬�ᵼ�²ⶨ����������ƫ�ⶨֵƫ����ȷ��
E���۲����ʱ���ζ�ǰ���ӣ��ζ����ӣ����²ⶨ����������ƫС���ⶨֵƫС���ʴ���
��ѡACD��
���� ���⿼����̽�����ʵ���ɼ��京���IJⶨ���ѶȽϴ�ע�����ʵ������������ܻ�����������IJ���������ע�����ʵ�����Ҫ���ע�����

 ��У����ϵ�д�
��У����ϵ�д�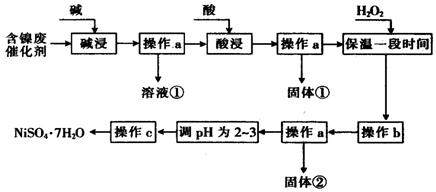
����������������������ʽ����ʱ��pH�����
| ������ | ��ʼ����ʱ��pH | ��ȫ����ʱ��pH |
| Al��OH��3 | 3.8 | 5.2 |
| Fe��OH��3 | 2.7 | 3.2 |
| Fe��OH��2 | 7.6 | 9.7 |
| Ai��OH��2 | 7.1 | 9.2 |
��2���������ʱ�����������H2SO4 ���ѧʽ����
��3������H2O2ʱ������Ӧ�����ӷ���ʽΪ2Fe2++2H++H2O2=2Fe3++2H2O
��4������bΪ������Һ��pH������ΪpH�ĵ��ط�Χ��3.2��PH��7.1��
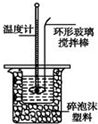 ijʵ��С����0.50mol/L NaOH��Һ��0.50mol/L������Һ�����к��ȵIJⶨ��
ijʵ��С����0.50mol/L NaOH��Һ��0.50mol/L������Һ�����к��ȵIJⶨ��������0.50mol/L NaOH��Һ
��1����ʵ���д�ԼҪʹ��245mL NaOH��Һ��������Ҫ����NaOH����5.0g
�ⶨϡ�����ϡ���������к��ȵ�ʵ��װ����ͼ��ʾ��
��1��д���÷�Ӧ���Ȼ�ѧ����ʽ���к���Ϊ57.3kJ/mol����$\frac{1}{2}$H2SO4��aq��+NaOH��aq��=$\frac{1}{2}$Na2SO4��aq��+H2O��l����H=-57.3kJ/mol��
��2��ȡ50mL NaOH��Һ��30mL������Һ����ʵ�飬ʵ�����������
������д���еĿհף�
| �¶� ʵ����� | ��ʼ�¶�t1/�� | ��ֹ�¶� t2/�� | �¶Ȳ�ƽ��ֵ ��t2-t1��/�� | ||
| H2SO4 | NaOH | ƽ��ֵ | |||
| 1 | 26.2 | 26.0 | 26.1 | 30.1 | |
| 2 | 27.0 | 27.4 | 27.2 | 33.3 | |
| 3 | 25.9 | 25.9 | 25.9 | 29.8 | |
| 4 | 26.4 | 26.2 | 26.3 | 30.4 | |
������ʵ����ֵ�����57.3kJ/mol��ƫ�����ƫ���ԭ�������acd������ĸ����
a��ʵ��װ�ñ��¡�����Ч���� b����ȡNaOH��Һ�����ʱ���Ӷ���
c���ֶ�ΰ�NaOH��Һ����ʢ�������С�ձ���
d�����¶ȼƲⶨNaOH��Һ��ʼ�¶Ⱥ�ֱ�ӲⶨH2SO4��Һ���¶ȣ�
CO��g��+H2O��g���TCO2��g��+H2��g����H=-41kJ/mol
ijС���о���ͬ�¶��·�Ӧ�����е������仯�����Ƿֱ��������ΪV L���������º����ܱ������м���һ�����ķ�Ӧ�ʹ������ͬ�¶��·�����Ӧ���������£�
| ������� | ��ʼʱ���������ʵ���/mol | �ﵽƽ���ʱ��/min | ��ƽ��ʱ��ϵ�����ı仯/kJ | ||||
| CO | H2O | CO2 | H2 | ||||
| �� | 1 | 4 | 0 | 0 | t1 | �ų�������32.8kJ | |
| �� | 2 | 8 | 0 | 0 | t2 | �ų�������Q | |
��2���������з�Ӧ��ƽ��ʱ��CO��ת����Ϊ80%��
��3�������������з�Ӧ��ƽ�ⳣ��K=1��
��4������������ȷ����a������ĸ��ţ���
a��ƽ��ʱ����������H2������������
b���������з�Ӧ��ƽ��״̬ʱ��Q��65.6kJ
c����Ӧ��ʼʱ���������з�Ӧ�Ļ�ѧ��Ӧ�������
d���������У���Ӧ�Ļ�ѧ��Ӧ����Ϊ��v��H2O��=4/Vt1mol/��L•min��
��5����֪��2H2��g��+O2��g���T2H2O��g����H=-484kJ/mol��
д��CO��ȫȼ������CO2���Ȼ�ѧ����ʽ��2CO��g��+O2��g����2CO2��g����H=-566 kJ/mol��
| ѹǿ/MPa �������/% �¶�/�� | 2.0 | 4.0 | 6.0 |
| 700 | 55.0 | a | b |
| 850 | c | 75.0 | d |
| 950 | e | f | 85.0 |
��2��a��b��e��f�Ĵ�С˳����e��f��a��b��
��3��ƽ�ⳣ���Ĵ�С��ϵ��K��700�棩��K��950�棩���������������
��4��850�桢4.0MPaʱA��ת����Ϊ60%��
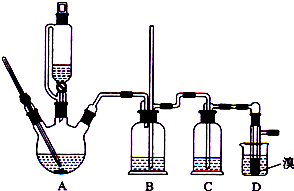
�й������б����£�
| �Ҵ� | 1��2-�������� | ���� | |
| ״̬ | ��ɫҺ�� | ��ɫҺ�� | ��ɫҺ�� |
| �ܶ�/g•cm-3 | 0.79 | 2.2 | 0.71 |
| �е�/�� | 78.5 | 132 | 34.6 |
| �۵�/�� | һl30 | 9 | -1l6 |
��1��д����ʵ������з�����������Ҫ��Ӧ�Ļ�ѧ����ʽCH3CH2OH $��_{170��}^{Ũ����}$CH2=CH2��+H2O��CH2=CH2+Br-Br��CH2Br-CH2Br��
��2���ڴ��Ƹ�ʵ���У�Ҫ������Ѹ�ٵذѷ�Ӧ�¶�170�����ң�������ҪĿ����d��������ȷѡ��ǰ����ĸ����ͬ��
a��������Ӧ b���ӿ췴Ӧ�ٶ� c����ֹ�Ҵ��ӷ� d�����ٸ�������������
��3����װ��C��Ӧ����c����Ŀ�������շ�Ӧ�п������ɵ��������壮
a��ˮ b��Ũ���� c������������Һ d������̼��������Һ
��4���жϸ��Ʊ���Ӧ�Ѿ�������������������ɫ��ȫ��ȥ����ʵ������
��5����1��2-��������ֲ�Ʒ���ڷ�Һ©���м�ˮ�����ã�����Ӧ���²�
��6����������������δ��Ӧ��Br2�������bϴ�ӳ�ȥ��
a��ˮ b������������Һ c���⻯����Һ d���Ҵ�
��7�������������������������ѣ���������ķ�����ȥ��
��8����Ӧ������Ӧ����ˮ��ȴװ��D������ҪĿ������ȴ�ɱ�����Ĵ����ӷ������ֲ��ܹ�����ȴ�����ñ�ˮ������ԭ���Dz�Ʒ1��2-����������۵㣨���̵㣩�ͣ�������ȴ�����̶��������ܣ�
| A�� | ��Ҫ���Ȳ��ܽ��еĻ�ѧ��Ӧһ�������ȷ�Ӧ | |
| B�� | ��ѧ��Ӧ�����仯�����������������ǹ��ܡ����ܵ� | |
| C�� | ��ѧ��Ӧ�����е������仯��Ҳ���������غ㶨�� | |
| D�� | ��Ӧ�������������������������ʱ���������ȷ�Ӧ |