��Ŀ����
X��Y��Z��W��R��Q��P��Ԫ�����ڱ��������еij���Ԫ�أ���ԭ���������������������Ϣ���±���
��1��R��Ԫ�����ڱ��е�λ��Ϊ ������Ԫ���У�ԭ�Ӱ뾶������ ����Ԫ�ط��ű�ʾ����
��2��Y������Ԫ�ذ�ԭ����Ŀ��1��3��2��4���ɷ���A��B��A�ĵ���ʽΪ�� ��B����H+��ϳ�Y2H5+����Y2H5+�ĽṹʽΪ�� ��������������
��3�����ж����л������̬�ľ������ͣ�������±���
��4���飨As��������������Ԫ�أ���Y��Qͬһ���壬Asԭ�ӱ�Qԭ�Ӷ�1�����Ӳ㣬����3��Ԫ�ص���̬�⻯����ȶ��ԴӴ�С��˳���� ���û�ѧʽ��ʾ����
��5��P�ĵ���������XP4�У������ǣ� ��
| Ԫ�� | �����Ϣ |
| X | ����һ�ַ�����ͬλ�أ�������һЩ�����ʯ������ⶨ |
| Z | ����������ϼۣ���������ϼ�Ϊ-2 |
| W | ����������Ϊ25��������Ϊ13�ĺ��� |
| R | λ�����ڱ���13�� |
| P | ��Z����ͬ�壬������������Ӧˮ����Ϊǿ�� |
��2��Y������Ԫ�ذ�ԭ����Ŀ��1��3��2��4���ɷ���A��B��A�ĵ���ʽΪ��
��3�����ж����л������̬�ľ������ͣ�������±���
| ������ | WZ | R2Z3 | WP2 | RP3 |
| �������� | ||||
| �۵�/�� | 2800 | 2050 | 714 | 191 |
��5��P�ĵ���������XP4�У������ǣ�
���㣺λ�ýṹ���ʵ����ϵӦ��
ר�⣺Ԫ����������Ԫ�����ڱ�ר��
������X������һ�ַ�����ͬλ�أ�������һЩ�����ʯ������ⶨ����XΪ̼��
Z����������ϼۣ���������ϼ�Ϊ-2����ZΪ����
X��Y��Zԭ��������������YΪ����
W��������Ϊ25��������Ϊ13�ĺ��أ�������Ϊ25-13=12����WΪþ��
Rλ�����ڱ���13�У���Ϊ��A��Ԫ�أ�����R��ԭ����������W����RΪ����
P��Z����ͬ�壬������������Ӧˮ����Ϊǿ�ᣬ��PΪ�ȣ��ݴ˽���С�⼴�ɣ�
Z����������ϼۣ���������ϼ�Ϊ-2����ZΪ����
X��Y��Zԭ��������������YΪ����
W��������Ϊ25��������Ϊ13�ĺ��أ�������Ϊ25-13=12����WΪþ��
Rλ�����ڱ���13�У���Ϊ��A��Ԫ�أ�����R��ԭ����������W����RΪ����
P��Z����ͬ�壬������������Ӧˮ����Ϊǿ�ᣬ��PΪ�ȣ��ݴ˽���С�⼴�ɣ�
���
�⣺X������һ�ַ�����ͬλ�أ�������һЩ�����ʯ������ⶨ����XΪ̼��
Z����������ϼۣ���������ϼ�Ϊ-2����ZΪ����
X��Y��Zԭ��������������YΪ����
W��������Ϊ25��������Ϊ13�ĺ��أ�������Ϊ25-13=12����WΪþ��
Rλ�����ڱ���13�У���Ϊ��A��Ԫ�أ�����R��ԭ����������W����RΪ����
P��Z����ͬ�壬������������Ӧˮ����Ϊǿ�ᣬ��PΪ�ȣ�
��1��RΪ����Al��Ԫ�����ڱ��е�λ��Ϊ�������ڢ�A�壻C��N��O��Mg��Al��Q��Cl��ͬһ�����У�ԭ������ԽС���뾶Խ��������Խ�뾶Խ��Mg����ʴ�Ϊ���������ڢ�A�壻Mg��
��2��N������Ԫ�ذ�ԭ����Ŀ��1��3��ɰ���A����2��4���ɷ�����B�������ĵ���ʽ ��������H+��ϳ�N2H5+����N2H5+�ĽṹʽΪ
��������H+��ϳ�N2H5+����N2H5+�ĽṹʽΪ ���ʴ�Ϊ
���ʴ�Ϊ ��
�� ��
��
��3��MgOΪ���Ӿ��壬Al2O3Ϊ���Ӿ��壬MgCl2Ϊ���Ӿ��壬AlCl3Ϊ���Ӿ��壬�ʴ�Ϊ�����Ӿ��壻���Ӿ��壻���Ӿ��壻���Ӿ��壻
��4���ǽ�����Խǿ����̬�⻯����ȶ���Խ�����ڷǽ�����N��P��As�����⻯���ȶ���˳��Ϊ��NH3��PH3��AsH3���ʴ�Ϊ��NH3��PH3��AsH3��
��5��Cl2��CCl4��Ϊ�Ǽ��Է��ӣ�������������ԭ����Cl2������CCl4�У��ʴ�Ϊ��Cl2��CCl4��Ϊ�Ǽ��Է��ӣ�������������ԭ����Cl2������CCl4�У�
Z����������ϼۣ���������ϼ�Ϊ-2����ZΪ����
X��Y��Zԭ��������������YΪ����
W��������Ϊ25��������Ϊ13�ĺ��أ�������Ϊ25-13=12����WΪþ��
Rλ�����ڱ���13�У���Ϊ��A��Ԫ�أ�����R��ԭ����������W����RΪ����
P��Z����ͬ�壬������������Ӧˮ����Ϊǿ�ᣬ��PΪ�ȣ�
��1��RΪ����Al��Ԫ�����ڱ��е�λ��Ϊ�������ڢ�A�壻C��N��O��Mg��Al��Q��Cl��ͬһ�����У�ԭ������ԽС���뾶Խ��������Խ�뾶Խ��Mg����ʴ�Ϊ���������ڢ�A�壻Mg��
��2��N������Ԫ�ذ�ԭ����Ŀ��1��3��ɰ���A����2��4���ɷ�����B�������ĵ���ʽ
 ��������H+��ϳ�N2H5+����N2H5+�ĽṹʽΪ
��������H+��ϳ�N2H5+����N2H5+�ĽṹʽΪ ���ʴ�Ϊ
���ʴ�Ϊ ��
�� ��
����3��MgOΪ���Ӿ��壬Al2O3Ϊ���Ӿ��壬MgCl2Ϊ���Ӿ��壬AlCl3Ϊ���Ӿ��壬�ʴ�Ϊ�����Ӿ��壻���Ӿ��壻���Ӿ��壻���Ӿ��壻
��4���ǽ�����Խǿ����̬�⻯����ȶ���Խ�����ڷǽ�����N��P��As�����⻯���ȶ���˳��Ϊ��NH3��PH3��AsH3���ʴ�Ϊ��NH3��PH3��AsH3��
��5��Cl2��CCl4��Ϊ�Ǽ��Է��ӣ�������������ԭ����Cl2������CCl4�У��ʴ�Ϊ��Cl2��CCl4��Ϊ�Ǽ��Է��ӣ�������������ԭ����Cl2������CCl4�У�
������������Ҫ�������Ԫ�ص��ƶ��Լ����������͵��жϡ��⻯���ȶ��Ե��ж��ȣ������е��⣮

��ϰ��ϵ�д�
�����Ŀ
������������ǿ����ʵ��ǣ�������
| A��H2CO3 |
| B��Cl2 |
| C��CaCO3 |
| D��NH3 |
����ȷ��ʾ���л�ѧ��Ӧ�����ӷ���ʽ�ǣ�������
| A��������NaHSO4��Ba��OH��2��Һ��Ӧ��Ba2++2OH-+2H++SO42-=BaSO4��+2H2O |
| B������þ��Һ������������Һ��Ӧ��SO42-+Ba2+=BaSO4�� |
| C��NH4HCO3��Һ�����NaOH��Һ��Ӧ��NH4++OH-=NH3��+H2O |
| D����̼��������Һ�еμ�����ϡ���CO32-+2H +=CO2�� +H2O |
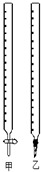 ��0.1320mol/L��HCl��Һ�ζ�δ֪Ũ�ȵ�NaOH��Һ��ʵ���������±���ʾ��
��0.1320mol/L��HCl��Һ�ζ�δ֪Ũ�ȵ�NaOH��Һ��ʵ���������±���ʾ��
