��Ŀ����
16��ij�����A����KAl��SO4��2��Al2O3��Fe2O3����һ�������¿�ʵ����ͼ��ʾ������֮���ת����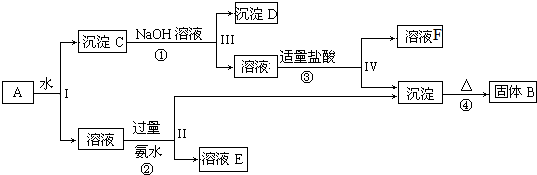
�ݴ˻ش��������⣺
��1�����IJ��ж�����Һ�ͳ����ķ����ȡ�ķ����ǹ��ˣ�
��2��������ͼ��Ӧ��ϵ��д������B��F�������ʳɷֵĻ�ѧʽ��BAl2O3�� FNaCl��
��3��д�����̷�Ӧ�ٵ����ӷ���ʽAl2O3+2OH-=2AlO2-+H2O������B������Ӧ�Ľ����䵥��������������ڸ����·�Ӧ�Ļ�ѧ����ʽ3Fe3O4+8Al$\frac{\underline{\;����\;}}{\;}$4Al2O3+9Fe��
��4��д�������������ʱ�����ӷ�Ӧ����ʽAlO2-+4H+=Al3++2H2O��AlO2-+4H+=Al3++2H2O��
���� �����̿�֪��Al2O3��Fe2O3������ˮ�������CΪAl2O3��Fe2O3������������Ӧ�������DΪFe2O3����Ӧ�ڢ������ɵij���ΪAl��OH��3�������������ȷֽ���������������BΪAl2O3����Ӧ��ΪKAl��SO4��2����ˮ�ķ�Ӧ������ҺEΪK2SO4����NH4��2SO4��NH3��H2O��Ȼ�������ʵ����ʷ������
��� �⣺�����̿�֪��Al2O3��Fe2O3������ˮ�������CΪAl2O3��Fe2O3������������Ӧ�������DΪFe2O3����Ӧ�ڢ������ɵij���ΪAl��OH��3�������������ȷֽ���������������BΪAl2O3����Ӧ��ΪKAl��SO4��2����ˮ�ķ�Ӧ������ҺEΪK2SO4����NH4��2SO4��NH3��H2O��
��1�����벻���Թ������Һ�ķ���Ϊ���ˣ����Ԣ��IJ��ж�����Һ�ͳ����ķ��뷽��Ϊ���ˣ��ʴ�Ϊ�����ˣ�
��2��������������֪��BΪAl2O3��FΪNaCl���ʴ�Ϊ��Al2O3��NaCl��
��3�����̷�Ӧ�������������������Ʒ�Ӧ��������ӷ���ʽ��Al2O3+2OH-=2AlO2-+H2O������B������Ӧ�Ľ����䵥��������������������������������ڸ����·�Ӧ�����ɵ�����������������Ӧ����ʽΪ��3Fe3O4+8Al $\frac{\underline{\;����\;}}{\;}$ 4Al2O3+9Fe���ʴ�Ϊ��Al2O3+2OH-=2AlO2-+H2O��3Fe3O4+8Al $\frac{\underline{\;����\;}}{\;}$ 4Al2O3+9Fe��
��4��ƫ����������ᷴӦ���������Ӻ�ˮ�����ӷ�Ӧ����ʽΪ��AlO2-+4H+=Al3++2H2O���ʴ�ΪAlO2-+4H+=Al3++2H2O����
���� ���⿼�������ʵķ�����ᴿ����ȷ���ʵ������ǽⱾ��ؼ���֪�������������������������Լ��ɽ�𣬸�����������������ȷ��ʵ������ȡ���������ķ������Ѷ��еȣ�

 ��У����ϵ�д�
��У����ϵ�д� CO��g��+H2O��g��?CO2��g��+H2��g����Ӧ�������仯��ͼ��ʾ�����з�Ӧ����������ԭ��Ӧ�����Ƿ��ȷ�Ӧ���ǣ�������
CO��g��+H2O��g��?CO2��g��+H2��g����Ӧ�������仯��ͼ��ʾ�����з�Ӧ����������ԭ��Ӧ�����Ƿ��ȷ�Ӧ���ǣ�������| A�� | þ����������ȼ�� | B�� | ľ̿��CO2��Ӧ | ||
| C�� | ���������������Һ��Ӧ | D�� | CO2��g��+H2��g��=CO��g��+H20��g�� |
| A�� | ��������Ca��OH��2���� | B�� | ��ˮϡ�� | ||
| C�� | ��������NaOH���� | D�� | ��������Na2CO3���� |
| Ԫ�ش���[ | X | Y | Z | W |
| ԭ�Ӱ뾶/nm | 0.130 | 0.118 | 0.102 | 0.073 |
| ��Ҫ���ϼ� | +2 | +3 | +6��-2 | -2 |
| A�� | X��YԪ�صĽ�����X��Y | |
| B�� | һ�������£�Z������W�ij�������ֱ������ZW2 | |
| C�� | Y������������Ӧ��ˮ����������ϡ��ˮ | |
| D�� | Z���⻯��ķе��W���⻯��� |
| A�� | ������������� | B�� | ����Ͱ�ˮ | C�� | ������������� | D�� | ������������� |
| A�� | pH=8 | B�� | pH=7 | C�� | pH=6 | D�� | pH���ӽ�7 |
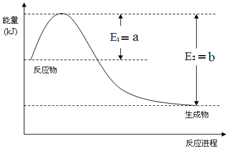 ���û�ѧ��Ӧԭ���о��������ȡ���ȵ��ʼ��仯����ķ�Ӧ����Ҫ����
���û�ѧ��Ӧԭ���о��������ȡ���ȵ��ʼ��仯����ķ�Ӧ����Ҫ����
 ��
�� ��
��