��Ŀ����
����������ȷ���ǣ�������
| A��0.01mol/L CH3COOH��pH=12��NaOH��Һ��ϣ�����Ϻ�c��CH3COO-����c��Na+��������Һһ���ʼ��� |
| B�������£��������0.01mol/L HCl��pH=12�İ�ˮ��ϣ�����Һ��pH=7 |
| C��0.1mol/L��ij��Ԫ������Na2A��Һ�У�c��Na+��=2c��H2A��+2c��HA-��+2c��A2-�� |
| D����5mL 0.02mol/L��H2SO4��5mL 0.02mol/L NaOH��Һ��ֻ�ϣ�����Ϻ���Һ�����Ϊ10mL������Һ��pH=7 |
���㣺�����ʱ�Ķ����жϼ��й�ph�ļ���,����ˮ���Ӧ��
ר�⣺����ƽ������Һ��pHר��,�����ˮ��ר��
������A��������Һ�е���غ�����жϣ�
B����ˮ��һˮ�ϰ���������ʣ���Һ�д��ڵ���ƽ�⣻
C��������Һ�������غ㼴Ԫ���غ��Ԫ�صĴ�����ʽ�Ͷ�����ϵn��Na����n��A��=2��1������
D����5mL 0.02mol/L��H2SO4��5mL 0.02mol/L NaOH��Һ��ֻ�ϣ������������������Һ��������Ũ�Ƚ����ҺpH��
B����ˮ��һˮ�ϰ���������ʣ���Һ�д��ڵ���ƽ�⣻
C��������Һ�������غ㼴Ԫ���غ��Ԫ�صĴ�����ʽ�Ͷ�����ϵn��Na����n��A��=2��1������
D����5mL 0.02mol/L��H2SO4��5mL 0.02mol/L NaOH��Һ��ֻ�ϣ������������������Һ��������Ũ�Ƚ����ҺpH��
���
�⣺A��������Һ�е���غ��������Һ�д��ڵ���غ㣬c��CH3COO-��+c��OH-��=c��Na+��+c��H+����������Ϻ�c��CH3COO-����c��Na+������c��OH-����c��H+�������Һһ�������ԣ���A����
B����ˮ��һˮ�ϰ���������ʣ���Һ�д��ڵ���ƽ�⣬�������0.01mol/L HCl��pH=12�İ�ˮ��ϣ�һˮ�ϰ��ֵ�������������ӣ�����Һ��pH��7����B����
C��������Һ�������غ㼴Ԫ���غ��Ԫ�صĴ�����ʽ�Ͷ�����ϵn��Na����n��A��=2��1������0.1mol/L��ij��Ԫ������Na2A��Һ�У�c��Na+��=2c��H2A��+2c��HA-��+2c��A2-������C��ȷ��
D����5mL 0.02mol/L��H2SO4��5mL 0.02mol/L NaOH��Һ��ֻ�ϣ���������������Һ��ʣ��������Ũ��=
=0.01mol/L����ҺPH=2����D����
��ѡC��
B����ˮ��һˮ�ϰ���������ʣ���Һ�д��ڵ���ƽ�⣬�������0.01mol/L HCl��pH=12�İ�ˮ��ϣ�һˮ�ϰ��ֵ�������������ӣ�����Һ��pH��7����B����
C��������Һ�������غ㼴Ԫ���غ��Ԫ�صĴ�����ʽ�Ͷ�����ϵn��Na����n��A��=2��1������0.1mol/L��ij��Ԫ������Na2A��Һ�У�c��Na+��=2c��H2A��+2c��HA-��+2c��A2-������C��ȷ��
D����5mL 0.02mol/L��H2SO4��5mL 0.02mol/L NaOH��Һ��ֻ�ϣ���������������Һ��ʣ��������Ũ��=
| 0.005L��0.02mol/L��2-0.02mol/L��0.005L |
| 0.01L |
��ѡC��
���������⿼���˵������Һ�еĵ���غ㡢�����غ�����жϣ���Ӧ����Һ����Եļ�������жϣ����ռ��㷽����������ʵ���ƽ������ǽ���ؼ�����Ŀ�Ѷ��еȣ�

��ϰ��ϵ�д�
�����Ŀ
����ͭ����Ҫ���ջ�ͭ���䷴ӦΪ��2CuFeS2+O2�TCu2S+2FeS+SO2����˵����ȷ���ǣ�������
| A��CuFeS2������ԭ������Ԫ�ر����� |
| B��SO2���������FeS�ǻ�ԭ���� |
| C��ÿ����1mol Cu2S����4mol������ |
| D��ÿת��1.2mol���ӣ���0.2mol������ |
NA���������ӵ�����������˵����ȷ���ǣ�������
| A��33.6L������27g����ַ�Ӧ��ת�Ƶĵ�����һ��Ϊ3NA |
| B����0.2mol Cl2�ܽ���10L��ˮ�У�������0.2NA��HClO |
| C����CO2��O2��ɵĻ�����й���NA�����ӣ����е���ԭ����Ϊ2NA |
| D��0.012kg 13C�У���̼ԭ����ΪNA |
NAΪ����٤��������ֵ������������ȷ���ǣ�������
| A��1.0L 1.0mo1?L-1��NaAlO2ˮ��Һ�к��е���ԭ����Ϊ2NA |
| B��12gʯīϩ������ʯī���к�����Ԫ���ĸ���Ϊ0.5NA |
| C��25��ʱpH=13��NaOH��Һ�к���OH-����ĿΪ0.1NA |
| D��1mol���ǻ���1moL������������������������Ϊ9NA |
��ͼ��ʾ��ʵ�飬�ܴﵽʵ��Ŀ���ǣ�������
| A | B | C | D |
 |
 |
 |
 |
| ��֤��ѧ��ת��Ϊ���� | ��֤�¶ȶ�ƽ���ƶ���Ӱ�� | ��֤�������ⸯʴ | ��֤�ǽ���Cl��C��Si |
| A��A | B��B | C��C | D��D |
�ڳ����µ�Al2��SO4��3��K2SO4��H2SO4�Ļ����Һ�У���pH=1��c��SO42-��=0.8mol/L��c��Al3+��=0.4mol/L����K+�����ʵ�Ũ��Ϊ��������
| A��0.1 mol/L |
| B��0.2 mol/L |
| C��0.3 mol/L |
| D��0.4 mol/L |


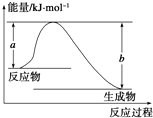 �Ͽ�1mol AB��g�������еĻ�ѧ��ʹ��ֱ�������̬Aԭ�Ӻ���̬Bԭ�������յ�������ΪA-B���ļ��ܣ��±��г���һЩ��ѧ���ļ���E��
�Ͽ�1mol AB��g�������еĻ�ѧ��ʹ��ֱ�������̬Aԭ�Ӻ���̬Bԭ�������յ�������ΪA-B���ļ��ܣ��±��г���һЩ��ѧ���ļ���E�� S2Cl2�����������л����Ȼ����Լ���ij�о���ѧϰС������ʵ���ҳ����Լ��������ϳ�S2Cl2��
S2Cl2�����������л����Ȼ����Լ���ij�о���ѧϰС������ʵ���ҳ����Լ��������ϳ�S2Cl2��