��Ŀ����
17��ҽ���Ȼ��ƿ������������ơ�������������ҩ��Թ�ҵ̼��ƣ���������Na+��Al3+��Fe3+�����ʣ�����ҽҩ����ˮ���Ȼ��ƣ�CaCl2•2H2O����������Ϊ97.0%��103.0%������Ҫ�������£�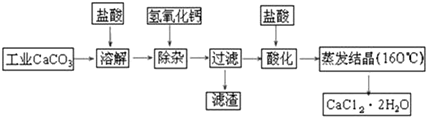
��1�����Ӳ����Ǽ����������ƹ��壬������Һ��pHΪ8.0��8.5���Գ�ȥ��Һ�е�����Al3+��Fe3+������Fe��OH��3�Ƿ������ȫ��ʵ�������ȡ�����ϲ���Һ���μ�KSCN��Һ����������Ѫ��ɫ�������Fe��OH��3������ȫ��
��2���ữ�����Ǽ������ᣬ������Һ��pHԼΪ4.0����Ŀ���У�
�ٽ���Һ�е�����Ca��OH��2ת��ΪCaCl2��
�ڷ�ֹ��Һ���տ�����CO2��
��3���ⶨ��Ʒ��Cl-�����ķ����ǣ�
a����ȡ0.7500g��Ʒ���ܽ⣬��250mL����ƿ�ж��ݣ�b����ȡ25.00mL������Һ����ƿ�У�c����0.0500mol•L-1AgNO3��Һ�ζ����յ㣬����AgNO3��Һ�����ƽ��ֵΪ20.00mL��
�������ⶨ�����в���Ҫ��ʢװ��Һ��ϴ����������ƿ������ϴ��ᵼ�½��ƫ��
�ڼ���������Ʒ��CaCl2•2H2O����������Ϊ99.9%��
���������������ⶨ����Ʒ��CaCl2•2H2O����������ƫ�ߣ��ⶨ�����в��������ɺ��ԣ��������ԭ���У���Ʒ�д���������NaCl��������CaCl2.2H2Oʧˮ��
��4����ҵ��ұ�������Ƶķ����ǵ�ⷨ����д����ұ���Ļ�ѧ��Ӧ����ʽCaCl2 $\frac{\underline{\;ͨ��\;}}{\;}$Ca+Cl2����
���� ��ҵ̼��Ƽ����������ȫ�ܽ������Ȼ��ơ��Ȼ����Լ��Ȼ����ȣ������Լ�����������Һ������Һ��pHΪ8.0��8.5���Գ�ȥ��Һ��������Al3+��Fe3+��Ȼ���ټ������ữ�������������������ᾧ�ɵõ�CaCl2•2H2O��
��1��Fe3+��KSCN��Ӧ���ɺ�ɫ����Fe��SCN��3������Fe3+�Ƿ���ڵģ�ѡ��KSCN��Һ��
��2�����ڳ��ӹ����м�����Ca��OH��2���ʼ�����������Һ�е�����Ca��OH��2��Ӧʹ��ת��ΪCaCl2����Ca��OH��2�����տ����е�CO2������CaCO3�������ʼ������ỹ���Է�ֹ��Һ���տ�����CO2��Ca��OH��2���տ����е�CO2������CaCO3������������մ�����CO2 �ᵼ��������������ƫ�ͣ�
��3���ٵζ�ʵ���еζ�����Ҫ�ô�װҺ��ϴ����ƿ����Ҫ��ϴ��
�ڸ��ݵ���ζ��յ������Ȼ��������ʵ����������ĵ������������ʵ�����һ��ϵ������������������ʵ�������n��AgCl��=2n��CaCl2.2H2O����
�ݴ˿������ʵ���ϵ�CaCl2.2H2O�����ʵ������������������ע����Һ����Ǵ�250mol��ȡ25ml�������ڼ���ʱҪע����һ�㣻
����Ʒ�д���������NaCl������ n��AgCl��2n��CaCl2.2H2O����֪��CaCl2.2H2O�����ʵ�������ͬ����CaCl2.2H2Oʧˮ���·�ĸ��С��ֵƫ��
��4������ұ���ķ�����Ҫ�У�
�ȷֽⷨ�����ڲ����ý���������ֱ���ü��ȷֽ�ķ������������仯�����л�ԭ������Hg����߽�������
�Ȼ�ԭ�����ڽ������˳����д����м�λ�õĽ�����ͨ�����û�ԭ����C��CO��H2�����ý����ȣ����������仯�����л�ԭ������Zn��Cu����
��ⷨ�����ý��������û�ԭ����ԭ��ͨ�����õ�����ڵĽ���������ķ���ұ�����ý�����K��Al����
��� �⣺��1��Fe3+��KSCN��Ӧ���ɺ�ɫ����Fe��SCN��3������Fe3+�Ƿ���ڵģ�ѡ��KSCN��Һ��
�ʴ�Ϊ��ȡ�����ϲ���Һ���μ�KSCN��Һ����������Ѫ��ɫ�������Fe��OH��3 ������ȫ��
��2���ữ�����Ǽ������ᣬ������Һ��pHԼΪ4.0����Ŀ���У�����Һ�е�����Ca��OH��2ת��ΪCaCl2���۷�ֹ��Һ���տ�����CO2��
�ʴ�Ϊ������Һ�е�����Ca��OH��2ת��ΪCaCl2����ֹ��Һ���տ�����CO2��
��3���ٵζ�ʵ���еζ�����Ҫ�ô�װҺ��ϴ����ƿ����Ҫ��ϴ������ϴ�����������Һ������������������������Һ������ⶨ����Һ��Ũ��ƫ�ߣ�
�ʴ�Ϊ����ƿ��ƫ�ߣ�
����Ʒ��n��Cl-��=0.05000mol•L-1��0.02039L��10=0.010195mol������n��AgCl��=2n��CaCl2.2H2O������n��CaCl2.2H2O��=0.0050975mol������m��CaCl2.2H2O��=0.0050975mol��147g/mol=0.7493325g�����У�$\frac{0.7493225g}{0.7500g}$��100%=99.9%��
�ʴ�Ϊ��99.9%��
����Ʒ�д���������NaCl�ᵼ��CaCl2.2H2O�����ʵ�������ͬ����CaCl2.2H2Oʧˮ���·�ĸ��С��ֵƫ��
�ʴ�Ϊ����Ʒ�д���������NaCl��������CaCl2.2H2Oʧˮ��
��4����ҵ��ұ�������Ƶķ����ǵ�������Ȼ��Ƶõ�����Ӧ�Ļ�ѧ����ʽΪ��CaCl2 $\frac{\underline{\;ͨ��\;}}{\;}$Ca+Cl2����
�ʴ�Ϊ����ⷨ�� CaCl2 $\frac{\underline{\;ͨ��\;}}{\;}$Ca+Cl2����
���� ���⿼�������к����IJⶨ���漰ʵ��Ļ���������ʵ��������ѡ��ʵ�����������������뼰�����ⶨ�ļ���ȣ�ע�����ӵļ��鷽���ͳ���������ʹ�ã���Ʒ���ȵķ���Ҫע����Һ�п��ܷ����ķ�Ӧ��ע����Ч�������⣬��Ŀ�ۺ��Խ�ǿ���Ѷ��еȣ�

| A�� | ̼��ĵ��뷽��ʽ��H2CO3=2H++CO32- | |
| B�� | F-�Ľṹʾ��ͼ��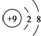 | |
| C�� | ${\;}_{55}^{134}$Cs��${\;}_{55}^{137}$Cs�γɵĵ�������������ͬ | |
| D�� | NH3 �ĵ���ʽ�� |
| A�� | HCl | B�� | CH3COONa | C�� | FeCl3 | D�� | NaCl |
| A�� | �����ʵ�Ũ��֮��c��A����c��B����c��C��=2��3��4 | |
| B�� | ƽ���������и����ʵ�Ũ����� | |
| C�� | ƽ������������Ƿ�Ӧ��ʼǰ��$\frac{4}{5}$ | |
| D�� | ��λʱ���ڣ���������a mol A���ʣ�ͬʱҲ������2a mol C���� |
| A�� | FeS | B�� | BaSO3 | C�� | BaSO4 | D�� | S |
��v��A��=0.15mol/��L•s��
��v��B��=0.6mol/��L•s��
��v��C��=0.5mol/��L•s��
��v��D��=0.45mol/��L•s��
��Ӧ�����ɿ쵽����˳��Ϊ��������
| A�� | �ܣ��ۣ��٣��� | B�� | �ܣ��ۣ��ڣ��� | C�� | �ڣ��ۣ��ܣ��� | D�� | �ڣ��ܣ��ۣ��� |
