��Ŀ����
14�������ȣ�SO2Cl2���������л���ѧ���Ȼ�������ҩ���Ⱦ�ϵ���ȡ��Ҳ����Ҫ���ã�ij��ѧѧϰС�����ø���������Ͷ��������ڻ���̿������ȡ�����ȣ�SO2��g��+Cl2��g���TSO2Cl2��l����H=-97.3kJ•mol-1��ʵ��װ����ͼ��ʾ�����ּг�װ��δ��������
��֪��������ͨ��������Ϊ��ɫҺ�壬�۵�-54.1�棬�е�69.1�森�ڳ�ʪ�����С����̡���100��C���Ͽ�ʼ�ֽ⣬���ɶ�����������������ڷ���Ҳ�ᷢ���ֽ⣮ �ش��������⣺
��1��ʢ�Ż���̿������������������ƿ����������ˮ�������a���a����b��������ο��Ʒ�Ӧ�������ȹ۲��ҡ������ܿڲ������ݵ��ٶ���ȣ�
��2����װ���Ϸ���Һ©�������ѡ������b�Լ���������ĸ��
a������ˮ b������ʳ��ˮ c��Ũ����������Һ d.6.0mol/L����
��3����ȱ��װ���ҺͶ�����ʢ��Ũ���ᣩ����ʪ�����Ͷ�����������Ӧ��ѧ����ʽ�� Cl2+SO2+2H2O=2HCl+H2SO4 ��
��4���Ȼ��ᣨClSO3H�����ȷֽ⣬Ҳ���Ƶ������ȣ�2ClSO3H=SO2Cl2+H2SO4���������ֲ���ķ�����c��������ȷѡ��ǰ����ĸ����
a���ؽᾧ b������ c������ d����ȡ
��5�����ڴ���������Ȼᷢ�ƣ����ܵ�ԭ����SO2Cl2=SO2+Cl2���ֽ���������������ܽ������У�ʹ�ñ�Ҫ���ֺ���ط���ʽ���Խ��ͣ���
��6������Ӧ�����ĵ��������Ϊ896mL����״���£�����������ᴿ�õ�4.05g�����������ȣ��������ȵIJ���Ϊ75%��Ϊ��߱�ʵ���������ȵIJ��ʣ���ʵ���������Ҫע��������Т٢ڢۣ�����ţ���
����ͨ����ˮ����ͨ�� �ڿ����������ʣ��������˿������װ�÷��̣����ʵ����� �ܼ��ȱ�װ�ã�
���� �ø���������Ͷ��������ڻ���̿������ȡ�����ȣ������������ƺ�Ũ�����Ʊ����������������ڻ���̿�����ڱ�װ���з�����Ӧ�Ʊ������ȣ��ҡ���ʢ��Ũ��������������������������Ͷ���������ֱ���ŷ��ڿ��������ü�װ�ü�ʯ�ҵĸ�������գ��ݴ˷�������
��� �⣺��1�������������֪ʢ�Ż���̿������Ϊ������ƿ������ˮ��������������Ч���Ϻã���aΪ��ڣ�ͨ���۲��ҡ������ܿڲ������ݵ��ٶ���ȿ��Ʒ�Ӧ�������ȣ�
�ʴ�Ϊ��������ƿ��a���۲��ҡ������ܿڲ������ݵ��ٶ���ȣ�
��2������ˮ�����ڱ���ʳ��ˮ����E�е��Լ��DZ���ʳ��ˮ��
�ʴ�Ϊ��b��
��3����ȱ��װ���ҺͶ�����ʢ��Ũ���ᣩ����ʪ�����Ͷ�����������Ӧ������ˮ���ڵ������¿ɽ�SO2���������ᣬ��������ԭΪHCl����Ӧ�ķ���ʽΪSO2+Cl2+2H2O=H2SO4+2HCl��
�ʴ�Ϊ��Cl2+SO2+2H2O=2HCl+H2SO4��
��4������Ϊ����Һ�壬�е����ϴ�ȡ�����з��룻
�ʴ�Ϊ��c��
��5�����ڴ���������Ȼᷢ�ƣ������֪�������ȷֽ⣺SO2Cl2=SO2+Cl2���ֽ���������������ܽ������У�
�ʴ�Ϊ��SO2Cl2=SO2+Cl2���ֽ���������������ܽ������У�
��6�����ĵ��������Ϊ896mL����״���£�������SO2��g��+Cl2��g���TSO2Cl2�������������ɵ�m��SO2Cl2��=$\frac{0.896L}{22.4L/mol}��135g/mol$=5.4g�������ȵIJ���Ϊ$\frac{4.05}{5.4}��100%$=75%��Ϊ��߱�ʵ���������ȵIJ��ʣ�������ͨ����ˮ����ͨ���������������٣��������˿죬ʹ���ַ�Ӧ������100������SO2Cl2��ʼ�ֽ⣬�÷�ӦΪ���ȷ�Ӧ�����Զ�������ƿ�����ʵ��Ľ��£�
�ʴ�Ϊ��75%���٢ڢۣ�
���� ���⿼��ʵ���Ʊ��������漰�Է�Ӧԭ����װ�ü������ķ������ۡ���������ʶ�����ʵķ����ᴿ�ȣ�ע�������������Ϣ��Ӧ�ã��Ѷ��еȣ�

| A�� | IA��Ԫ�صĽ����Ա�IIA��Ԫ�صĽ�����ǿ | |
| B�� | HF��HCl��HBr��HI�Ļ�ԭ�Դ�����������ǿ | |
| C�� | ͬ���ڷǽ����������Ӧ��ˮ��������Դ�����������ǿ | |
| D�� | ��������Ԫ�ص����Ӱ뾶��������С |

��1��װ��A�з�Ӧ�����ӷ���ʽMnO2+4H++2Cl-$\frac{\underline{\;\;��\;\;}}{\;}$Mn2++Cl2��+2H2O��
��2������װ������˳��ΪAACFBDECACFBDEF��
��3��װ��A��a�ܵ������ǵ�����װ��B�б���ʳ��ˮ���������������е��Ȼ������壮
��4����֪�����ᣨH3BO3����һԪ���ᣬ�����λ�ѧʽΪNa[B��OH��4]����������ˮ�еĵ��뷽��ʽ��H3BO3+H2O?[B��OH��4]-+H+��
��5��ʵ����ɺ�С��ͬѧ��F�У���Һ����NaClO��NaCl��NaOH���μ�Ʒ����Һ��������Һ��ɫ������ƶ���ʵ��̽����Һ��ɫ��Ӱ�����أ�
| ʵ����� | 0.1mol/LNaClO��Һ/mL | 0.1mol/LNaCl��Һ/mL | 0��mol/LNaOH��Һ/mL | H2O/mL | Ʒ����Һ | ���� |
| �� | 4.0 | 2.0 | 0 | 2.0 | 3�� | �Ͽ���ɫ |
| �� | 4.0 | 4.0 | 0 | 0 | 3�� | ������ɫ |
| �� | 4.0 | 0 | 4.0 | 0 | 3�� | ������ɫ |
| �� | 4.0 | 0 | 2.0 | b | 3�� | �Ͽ���ɫ |
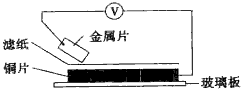 ���ྻ�Ľ���Ƭ�ס��ҡ��������ֱ�����ڽ���ij������Һ����ֽ���沢ѹ������ͼ��ʾ������ÿ��ʵ��ʱ����¼��ѹָ����ƶ�����͵�ѹ���Ķ������
���ྻ�Ľ���Ƭ�ס��ҡ��������ֱ�����ڽ���ij������Һ����ֽ���沢ѹ������ͼ��ʾ������ÿ��ʵ��ʱ����¼��ѹָ����ƶ�����͵�ѹ���Ķ������ | ���� | ������������ | ��ѹ |
| �� | �ס�Cu | +0.78 |
| �� | Cu���� | +0.15 |
| �� | ����Cu | +1.35 |
| �� | ����Cu | +0.30 |
| A�� | �����ֽ������ҵĻ�ԭ����ǿ | |
| B�� | �������ܴ�����ͭ��Һ���û���ͭ | |
| C�� | �ס������γ�ԭ���ʱ����Ϊ���� | |
| D�� | �ס����γɺϽ��ڿ����У����ȱ���ʴ |
| ѡ�� | ������ | X�Լ� | ���ӷ���ʽ |
| A | K+��Na+��ClO-��SO42- | ����SO2 | SO2+ClO-+H2O�TSO42-+Cl-+2H+ |
| B | NH4+��Fe3+��Br-��SO42- | ����H2S | 2Fe3++H2S�T2Fe2++S��+2H+ |
| C | NH4+��Na+��Fe3+��AlO2- | ����ͭ�� | 2Fe3++Cu�T2Fe2++Cu2+ |
| D | K+��Na+��HCO3-��AlO2- | ����HCl | H++AlO2-+H2O�TAl��OH��3�� |
| A�� | A | B�� | B | C�� | C | D�� | D |
 -CH=CH-CH3���й���ṹ˵����ȷ���ǣ�������
-CH=CH-CH3���й���ṹ˵����ȷ���ǣ�������| A�� | ����ԭ�ӿ�����ͬһƽ���� | B�� | ��������̼ԭ�ӿ��ܹ�ֱ�� | ||
| C�� | ����̼ԭ�ӿ��ܹ��� | D�� | �����18ԭ�ӹ��� |