��Ŀ����
����ѧ--ѡ��5�л���ѧ������
I������ƽF�ǽ�Ѫ֬�������̴���ҩ�����һ���ϳ�·�����£�

��֪��RCH2COOH
 ��
�� +RCl��
+RCl�� +NaCl
+NaCl
��1��AΪһԪ���ᣬ8.8gA������NaHCO3��Һ��Ӧ���� 2.24LCO2����״������A�ķ���ʽΪ ��
��2��B���ȴ����ᣬ��˴Ź��������������壬д��B��C�ķ�Ӧ�Ļ�ѧ����ʽ�� ��
��3��D�Ĺ����ŵ�����Ϊ �� b���������Լ��� ��
��4��C+E��F�ķ�Ӧ����Ϊ �� F�Ľṹ��ʽ�� ��
��5��C������ȥ��Ӧ�IJ���G�ж���ͬ���칹�壬�����������������Ľṹ�� �֣���������˳���칹��
����G������ͬ������ ���ܷ���������Ӧ �۽ṹ��ֻ��һ������
I������ƽF�ǽ�Ѫ֬�������̴���ҩ�����һ���ϳ�·�����£�

��֪��RCH2COOH
| Cl2 |
| ����(����) |
 ��
�� +RCl��
+RCl�� +NaCl
+NaCl��1��AΪһԪ���ᣬ8.8gA������NaHCO3��Һ��Ӧ���� 2.24LCO2����״������A�ķ���ʽΪ
��2��B���ȴ����ᣬ��˴Ź��������������壬д��B��C�ķ�Ӧ�Ļ�ѧ����ʽ��
��3��D�Ĺ����ŵ�����Ϊ
��4��C+E��F�ķ�Ӧ����Ϊ
��5��C������ȥ��Ӧ�IJ���G�ж���ͬ���칹�壬�����������������Ľṹ��
����G������ͬ������ ���ܷ���������Ӧ �۽ṹ��ֻ��һ������
���㣺�л�����ƶ�
ר�⣺�л���Ļ�ѧ���ʼ��ƶ�
������AΪһԪ���ᣬ8.8gA������NaHCO3��Һ��Ӧ���� 2.24LCO2����״������������̼Ϊ0.1mol����AΪ0.1mol����A����Է�������Ϊ
=88��ȥ��1��-COOH��ʣ�����ʽ��Ϊ88-45=43����ʣ�����Ϊ-C3H7����AΪC3H7-COOH�������ʽΪC4H8O2��A����������ȡ����Ӧ����B��B���ȴ����ᣬ�Һ˴Ź��������������壬���Ƴ�BΪ ����AΪ
����AΪ ����C�Ľṹ��ʽΪ
����C�Ľṹ��ʽΪ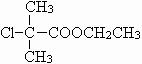 ���ɱ��ӡ�D��Eת������ϱ��ӡ�E�Ľṹ��ʽ��֪��DΪ
���ɱ��ӡ�D��Eת������ϱ��ӡ�E�Ľṹ��ʽ��֪��DΪ ���Լ�aΪ������Dת��bʱ���ǻ�������Ӧ�����Լ�b����ΪNa2CO3��Һ��C��F����ȡ����Ӧ����FΪ
���Լ�aΪ������Dת��bʱ���ǻ�������Ӧ�����Լ�b����ΪNa2CO3��Һ��C��F����ȡ����Ӧ����FΪ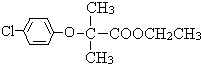 ���ݴ˽��
���ݴ˽��
| 8.8 |
| 0.1 |
 ����AΪ
����AΪ ����C�Ľṹ��ʽΪ
����C�Ľṹ��ʽΪ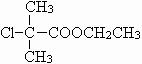 ���ɱ��ӡ�D��Eת������ϱ��ӡ�E�Ľṹ��ʽ��֪��DΪ
���ɱ��ӡ�D��Eת������ϱ��ӡ�E�Ľṹ��ʽ��֪��DΪ ���Լ�aΪ������Dת��bʱ���ǻ�������Ӧ�����Լ�b����ΪNa2CO3��Һ��C��F����ȡ����Ӧ����FΪ
���Լ�aΪ������Dת��bʱ���ǻ�������Ӧ�����Լ�b����ΪNa2CO3��Һ��C��F����ȡ����Ӧ����FΪ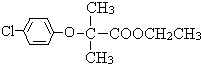 ���ݴ˽��
���ݴ˽�����
�⣺AΪһԪ���ᣬ8.8gA������NaHCO3��Һ��Ӧ���� 2.24LCO2����״������������̼Ϊ0.1mol����AΪ0.1mol����A����Է�������Ϊ
=88��ȥ��1��-COOH��ʣ�����ʽ��Ϊ88-45=43����ʣ�����Ϊ-C3H7����AΪC3H7-COOH�������ʽΪC4H8O2��A����������ȡ����Ӧ����B��B���ȴ����ᣬ�Һ˴Ź��������������壬���Ƴ�BΪ ����AΪ
����AΪ ����C�Ľṹ��ʽΪ
����C�Ľṹ��ʽΪ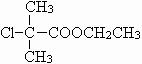 ���ɱ��ӡ�D��Eת������ϱ��ӡ�E�Ľṹ��ʽ��֪��DΪ
���ɱ��ӡ�D��Eת������ϱ��ӡ�E�Ľṹ��ʽ��֪��DΪ ���Լ�aΪ������Dת��bʱ���ǻ�������Ӧ�����Լ�b����ΪNa2CO3��Һ��C��F����ȡ����Ӧ����FΪ
���Լ�aΪ������Dת��bʱ���ǻ�������Ӧ�����Լ�b����ΪNa2CO3��Һ��C��F����ȡ����Ӧ����FΪ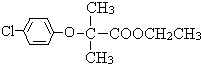 ��
��
��1��������������֪��A�ķ���ʽΪC4H8O2���ʴ�Ϊ��C4H8O2��
��2��B��C�ķ�Ӧ�Ļ�ѧ����ʽΪ�� ��
��
�ʴ�Ϊ�� ��
��
��3��DΪ �����й����ŵ�����Ϊ����ԭ�ӡ����ǻ��� b���������Լ���Na2CO3��Һ��
�����й����ŵ�����Ϊ����ԭ�ӡ����ǻ��� b���������Լ���Na2CO3��Һ��
�ʴ�Ϊ����ԭ�ӡ����ǻ���Na2CO3��Һ��
��4��C+E��F�ķ�Ӧ�ɿ���F��Clԭ�ӱ� ȡ����������������֪��F�Ľṹ��ʽΪ��
ȡ����������������֪��F�Ľṹ��ʽΪ��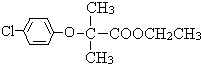 ���ʴ�Ϊ��ȡ����Ӧ��
���ʴ�Ϊ��ȡ����Ӧ��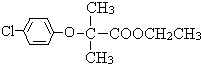 ��
��
��5��C��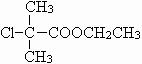 ��������ȥ��Ӧ�IJ���GΪCH2=C��CH3��COOCH2CH3��G�ж���ͬ���칹�壬������������������ͬ���칹�壺��G������ͬ�����ţ��ܷ���������Ӧ��Ӧ���м����γɵ��������ҽṹ��ֻ��һ����������������ͬ���칹���У�HCOOCH2CH2CH=CHCH3��HCOOCH2CH=CHCH2CH3��HCOOCH=CHCH2CH3��HCOOCH2CH2C��CH3��=CH2��HCOOCH2CH��CH3��CH=CH2��HCOOCH��CH3��CH2CH=CH2��HCOOCH2C��CH2CH3��=CH2��HCOOCH��CH2CH3��CH=CH2������һ����8�֣��ʴ�Ϊ��8��
��������ȥ��Ӧ�IJ���GΪCH2=C��CH3��COOCH2CH3��G�ж���ͬ���칹�壬������������������ͬ���칹�壺��G������ͬ�����ţ��ܷ���������Ӧ��Ӧ���м����γɵ��������ҽṹ��ֻ��һ����������������ͬ���칹���У�HCOOCH2CH2CH=CHCH3��HCOOCH2CH=CHCH2CH3��HCOOCH=CHCH2CH3��HCOOCH2CH2C��CH3��=CH2��HCOOCH2CH��CH3��CH=CH2��HCOOCH��CH3��CH2CH=CH2��HCOOCH2C��CH2CH3��=CH2��HCOOCH��CH2CH3��CH=CH2������һ����8�֣��ʴ�Ϊ��8��
| 8.8 |
| 0.1 |
 ����AΪ
����AΪ ����C�Ľṹ��ʽΪ
����C�Ľṹ��ʽΪ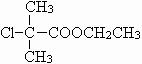 ���ɱ��ӡ�D��Eת������ϱ��ӡ�E�Ľṹ��ʽ��֪��DΪ
���ɱ��ӡ�D��Eת������ϱ��ӡ�E�Ľṹ��ʽ��֪��DΪ ���Լ�aΪ������Dת��bʱ���ǻ�������Ӧ�����Լ�b����ΪNa2CO3��Һ��C��F����ȡ����Ӧ����FΪ
���Լ�aΪ������Dת��bʱ���ǻ�������Ӧ�����Լ�b����ΪNa2CO3��Һ��C��F����ȡ����Ӧ����FΪ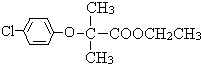 ��
����1��������������֪��A�ķ���ʽΪC4H8O2���ʴ�Ϊ��C4H8O2��
��2��B��C�ķ�Ӧ�Ļ�ѧ����ʽΪ��
 ��
���ʴ�Ϊ��
 ��
����3��DΪ
 �����й����ŵ�����Ϊ����ԭ�ӡ����ǻ��� b���������Լ���Na2CO3��Һ��
�����й����ŵ�����Ϊ����ԭ�ӡ����ǻ��� b���������Լ���Na2CO3��Һ���ʴ�Ϊ����ԭ�ӡ����ǻ���Na2CO3��Һ��
��4��C+E��F�ķ�Ӧ�ɿ���F��Clԭ�ӱ�
 ȡ����������������֪��F�Ľṹ��ʽΪ��
ȡ����������������֪��F�Ľṹ��ʽΪ��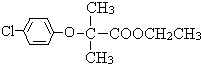 ���ʴ�Ϊ��ȡ����Ӧ��
���ʴ�Ϊ��ȡ����Ӧ��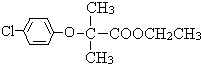 ��
����5��C��
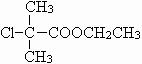 ��������ȥ��Ӧ�IJ���GΪCH2=C��CH3��COOCH2CH3��G�ж���ͬ���칹�壬������������������ͬ���칹�壺��G������ͬ�����ţ��ܷ���������Ӧ��Ӧ���м����γɵ��������ҽṹ��ֻ��һ����������������ͬ���칹���У�HCOOCH2CH2CH=CHCH3��HCOOCH2CH=CHCH2CH3��HCOOCH=CHCH2CH3��HCOOCH2CH2C��CH3��=CH2��HCOOCH2CH��CH3��CH=CH2��HCOOCH��CH3��CH2CH=CH2��HCOOCH2C��CH2CH3��=CH2��HCOOCH��CH2CH3��CH=CH2������һ����8�֣��ʴ�Ϊ��8��
��������ȥ��Ӧ�IJ���GΪCH2=C��CH3��COOCH2CH3��G�ж���ͬ���칹�壬������������������ͬ���칹�壺��G������ͬ�����ţ��ܷ���������Ӧ��Ӧ���м����γɵ��������ҽṹ��ֻ��һ����������������ͬ���칹���У�HCOOCH2CH2CH=CHCH3��HCOOCH2CH=CHCH2CH3��HCOOCH=CHCH2CH3��HCOOCH2CH2C��CH3��=CH2��HCOOCH2CH��CH3��CH=CH2��HCOOCH��CH3��CH2CH=CH2��HCOOCH2C��CH2CH3��=CH2��HCOOCH��CH2CH3��CH=CH2������һ����8�֣��ʴ�Ϊ��8��
���������⿼���л����ƶϣ����ؿ���ѧ���������ƶ���������ȷ���ʵĹ����ż��������ǽⱾ��ؼ�����A�ĽṹΪͻ�ƿڽ����ƶϣ�ע���������Ϣ���ѵ���ͬ���칹��������жϣ���Ŀ�ѵ��еȣ�

��ϰ��ϵ�д�
�����Ŀ
��Ũ�Ⱦ�Ϊ6mol?L-1������������100mL��Һ�У��ֱ��������������ۣ���ַ�Ӧ���������������Ϊ2��3����������۵������ǣ�������
| A��11.2 g |
| B��25.2 g |
| C��16.8 g |
| D��33.6 g |
������������ǿ����ʵ��ǣ�������
| A��H2CO3 |
| B��Cl2 |
| C��CaCO3 |
| D��NH3 |
����ȷ��ʾ���л�ѧ��Ӧ�����ӷ���ʽ�ǣ�������
| A��������NaHSO4��Ba��OH��2��Һ��Ӧ��Ba2++2OH-+2H++SO42-=BaSO4��+2H2O |
| B������þ��Һ������������Һ��Ӧ��SO42-+Ba2+=BaSO4�� |
| C��NH4HCO3��Һ�����NaOH��Һ��Ӧ��NH4++OH-=NH3��+H2O |
| D����̼��������Һ�еμ�����ϡ���CO32-+2H +=CO2�� +H2O |
 ��0.1320mol/L��HCl��Һ�ζ�δ֪Ũ�ȵ�NaOH��Һ��ʵ���������±���ʾ��
��0.1320mol/L��HCl��Һ�ζ�δ֪Ũ�ȵ�NaOH��Һ��ʵ���������±���ʾ�� ʵ��С����������KMnO4��Һ��H2C2O4��Һ��Ӧ�о�Ӱ�췴Ӧ���ʵ����أ�
ʵ��С����������KMnO4��Һ��H2C2O4��Һ��Ӧ�о�Ӱ�췴Ӧ���ʵ����أ�