��Ŀ����
ˮú�������Ǻϳɰ���ԭ������Ҳ�Ǻϳ������仯����Ʒ��ԭ�ϣ�
��1����ֱ��ˮú��ȼ�ϵ�ء��У�ͨCO��H2�ļ�Ϊ��ص� ����ѡ�������������������
��2��ˮú���任��Ӧ��CO��g��+H2O��g��?CO2��g��+H2��g����H��0�����д�ʩ����߷�Ӧ���ʵ��� ��������ѡ��
a�������¶� b��������� c������ѹǿ d������Ũ��
��3��H2��N2�ڴ��������¸�ѹ�����ºϳɰ��Ļ�ѧ����ʽΪ ��
��4��������ˮ��Һ�����������̵����еĶ������÷�Ӧ�Ļ�ѧ����ʽΪ ��
��5������״����582.4L�ϳ�������֪��n��CO����n��H2��=4��9��ͨ��ϳ�����һ�������¿ɷ���2CO��g��+4H2��g����CH2=CH2��g��+2H2O��g����CO��g��+3H2��CH4��g��+H2O��g������ַ�Ӧ���ⶨ��Ʒ��ֻ�м��顢��ϩ��ˮ�������ٶ�CO��H2����ʣ�ࣩ���Լ����ݳ�����������ϩ�����ʵ������г�������̣���
��1����ֱ��ˮú��ȼ�ϵ�ء��У�ͨCO��H2�ļ�Ϊ��ص�
��2��ˮú���任��Ӧ��CO��g��+H2O��g��?CO2��g��+H2��g����H��0�����д�ʩ����߷�Ӧ���ʵ���
a�������¶� b��������� c������ѹǿ d������Ũ��
��3��H2��N2�ڴ��������¸�ѹ�����ºϳɰ��Ļ�ѧ����ʽΪ
��4��������ˮ��Һ�����������̵����еĶ������÷�Ӧ�Ļ�ѧ����ʽΪ
��5������״����582.4L�ϳ�������֪��n��CO����n��H2��=4��9��ͨ��ϳ�����һ�������¿ɷ���2CO��g��+4H2��g����CH2=CH2��g��+2H2O��g����CO��g��+3H2��CH4��g��+H2O��g������ַ�Ӧ���ⶨ��Ʒ��ֻ�м��顢��ϩ��ˮ�������ٶ�CO��H2����ʣ�ࣩ���Լ����ݳ�����������ϩ�����ʵ������г�������̣���
���㣺��ѧƽ��ļ���,��ѧ��Դ���͵��,��ѧ��Ӧ���ʵ�Ӱ������
ר�⣺�����������������
��������1������ȼ�ϵ����ȼ���ڸ���ʧ���ӷ���������Ӧ��
��2������Ӱ�췴Ӧ���ʵ����ط����жϣ�
��3��H2��N2�ڴ��������¸�ѹ�����ºϳɰ��������ԭ���غ���ƽ��д��
��4��������ˮ��Һ�����������̵����еĶ�����������������炙���������泥�
��5������Ԫ���غ��ϻ�ѧ����ʽ������ϵ�������Ӧ�ã�
��2������Ӱ�췴Ӧ���ʵ����ط����жϣ�
��3��H2��N2�ڴ��������¸�ѹ�����ºϳɰ��������ԭ���غ���ƽ��д��
��4��������ˮ��Һ�����������̵����еĶ�����������������炙���������泥�
��5������Ԫ���غ��ϻ�ѧ����ʽ������ϵ�������Ӧ�ã�
���
�⣺��1��ȼ�ϵ����ȼ���ڸ���ʧ���ӷ���������Ӧ������ֱ��ˮú��ȼ�ϵ�ء��У�ͨCO��H2�ļ�Ϊ��صĸ������ʴ�Ϊ������
��2��CO��g��+H2O��g��?CO2��g��+H2��g����H��0����Ӧ�������������ķ��ȷ�Ӧ��
a�������¶�ƽ��������У���Ӧ��������a��ȷ��
b���������������Ӧ���ʣ���b��ȷ��
c������ѹǿ����Ӧ����������ȷ��
d������Ũ�ȣ���Ӧ���ʼ�С����d����
�ʴ�Ϊ��abc��
��3��H2��N2�ڴ��������¸�ѹ�����ºϳɰ��Ļ�ѧ����ʽΪ��N2+3H2
2NH3���ʴ�Ϊ��N2+3H2
2NH3��
��4��������ˮ��Һ�����������̵����еĶ�����������������炙���������泥���Ӧ�Ļ�ѧ����ʽΪ��SO2+2NH3+H2O=��NH4��2SO3��SO2+NH3+H2O=NH4HSO3��
�ʴ�Ϊ��SO2+2NH3+H2O=��NH4��2SO3��SO2+NH3+H2O=NH4HSO3��
��5���������⣺n��CO��+n��H2��=
=26mol��
n��CO��=26mol��4/��4+9��=8mol��n��H2��=26mol-8mol=18mol
�ɷ���ʽ��2CO��g��+4H2��g����C2H4��g��+2H2O��g����CO��g��+3H2��g����CH4��g��+H2O��g��
n��CH4��+2n��C2H4��=8mol��3n��CH4��+4n��C2H4��=18mol�����n��C2H4��=3mol��
���ݳ�����������ϩ�����ʵ���Ϊ3mol��
��2��CO��g��+H2O��g��?CO2��g��+H2��g����H��0����Ӧ�������������ķ��ȷ�Ӧ��
a�������¶�ƽ��������У���Ӧ��������a��ȷ��
b���������������Ӧ���ʣ���b��ȷ��
c������ѹǿ����Ӧ����������ȷ��
d������Ũ�ȣ���Ӧ���ʼ�С����d����
�ʴ�Ϊ��abc��
��3��H2��N2�ڴ��������¸�ѹ�����ºϳɰ��Ļ�ѧ����ʽΪ��N2+3H2
| ||
| ���¸�ѹ |
| ||
| ���¸�ѹ |
��4��������ˮ��Һ�����������̵����еĶ�����������������炙���������泥���Ӧ�Ļ�ѧ����ʽΪ��SO2+2NH3+H2O=��NH4��2SO3��SO2+NH3+H2O=NH4HSO3��
�ʴ�Ϊ��SO2+2NH3+H2O=��NH4��2SO3��SO2+NH3+H2O=NH4HSO3��
��5���������⣺n��CO��+n��H2��=
| 582.4L |
| 22.4L/mol |
n��CO��=26mol��4/��4+9��=8mol��n��H2��=26mol-8mol=18mol
�ɷ���ʽ��2CO��g��+4H2��g����C2H4��g��+2H2O��g����CO��g��+3H2��g����CH4��g��+H2O��g��
n��CH4��+2n��C2H4��=8mol��3n��CH4��+4n��C2H4��=18mol�����n��C2H4��=3mol��
���ݳ�����������ϩ�����ʵ���Ϊ3mol��
���������⿼���˻�ѧ��Ӧ���ʣ���ѧƽ���Ӱ�����ط����жϣ���ѧ����ʽ��д��ԭ���ԭ������Ӧ�ã����ջ����ǹؼ�����Ŀ�ϼ�

��ϰ��ϵ�д�
 ѧ�����νӽ̲��Ͼ���ѧ������ϵ�д�
ѧ�����νӽ̲��Ͼ���ѧ������ϵ�д�
�����Ŀ
����ʵ�����������ⶨ���ƫ�ߵ��ǣ�������
| A������֪Ũ�ȵ�����ζ�δ֪Ũ�ȵ�����������Һʱ����ȡ�ζ���ĩ����ʱ�����ӿ̶��� |
| B���ⶨ����ͭ�����нᾧˮ������ʵ��ʱ������ʱ�����δ��ȫ��� |
| C���к͵ζ�ʱ���Ӵ���Һǰ��ƿ��������ˮ |
| D���ⶨ1mol��������IJ����У���Ӧ������δ���� |
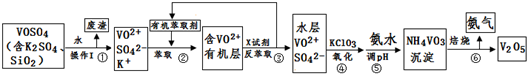
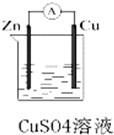 ij��ѧ��ȤС��������ͼ��ʾԭ���װ�ý���ʵ�飬��ش��������⣺
ij��ѧ��ȤС��������ͼ��ʾԭ���װ�ý���ʵ�飬��ش��������⣺