��Ŀ����
��9mol/L��Ũ����ϡ�ͳ�0.9mol/L��ϡ����100mL���ش��������⣺
��1����ҪȡŨ���� mL
��2�����Ʋ����ɷֽ�����¼�����������ȷ�IJ���˳����
A��������ƿ��ע����������ˮ������Ƿ�©ˮ
B������������ˮϴ���ձ���������������Һע������ƿ�����ظ���������
C��������ȴ��ϡ����ע���Ѽ�鲻©ˮ������ƿ��
D�����ݼ��㣬����Ͳ��ȡһ�������Ũ����
E����Ũ�������ձ�������ע��ʢ������ˮ��С�ձ��У��������ò���������
F����������ƿ���ӣ���ҡ��
G���ý�ͷ�ιܵμ�����ˮ��ʹ��Һ����ǡ����̶�����
H������������ƿ��С�ĵؼ�����ˮ��ʹҺ��ӽ��̶���1��2cm
��3����Ҫʹ�õ�ʵ���������ձ� ��
��4�����ڴ��������ʹ�õ���Ũ�����ݱ���ȷ��ƫ����� ����д��ţ���
A��ʹ������ƿ������Һʱ������Һ�涨�ݺ�������Һ��Ũ��
B��û��������ˮϴ�ձ�2-3�Σ�����ϴҺ��������ƿ��
C������ƿ������ˮϴ����û�к��
D������ʱ���μ�����ˮ����ʹҺ���Ը��ڿ̶��ߣ�����������ˮʹҺ�氼����̶�������
E������õ���Һ����������ˮϴ����ĩ�ɵ��Լ�ƿ�б��ã�
��1����ҪȡŨ����
��2�����Ʋ����ɷֽ�����¼�����������ȷ�IJ���˳����
A��������ƿ��ע����������ˮ������Ƿ�©ˮ
B������������ˮϴ���ձ���������������Һע������ƿ�����ظ���������
C��������ȴ��ϡ����ע���Ѽ�鲻©ˮ������ƿ��
D�����ݼ��㣬����Ͳ��ȡһ�������Ũ����
E����Ũ�������ձ�������ע��ʢ������ˮ��С�ձ��У��������ò���������
F����������ƿ���ӣ���ҡ��
G���ý�ͷ�ιܵμ�����ˮ��ʹ��Һ����ǡ����̶�����
H������������ƿ��С�ĵؼ�����ˮ��ʹҺ��ӽ��̶���1��2cm
��3����Ҫʹ�õ�ʵ���������ձ�
��4�����ڴ��������ʹ�õ���Ũ�����ݱ���ȷ��ƫ�����
A��ʹ������ƿ������Һʱ������Һ�涨�ݺ�������Һ��Ũ��
B��û��������ˮϴ�ձ�2-3�Σ�����ϴҺ��������ƿ��
C������ƿ������ˮϴ����û�к��
D������ʱ���μ�����ˮ����ʹҺ���Ը��ڿ̶��ߣ�����������ˮʹҺ�氼����̶�������
E������õ���Һ����������ˮϴ����ĩ�ɵ��Լ�ƿ�б��ã�
���㣺����һ�����ʵ���Ũ�ȵ���Һ
ר�⣺���ʵ���Ũ�Ⱥ��ܽ��ר��
��������1��������Һ��ϡ���������ʵ����ʵ�������������ҪŨ����������
��2����������100mL 0.9mol/L��ϡ����IJ���Ը�������������
��3����������һ�����ʵ���Ũ�ȵIJ���ѡ������
��4������c=
�ɵã�һ�����ʵ���Ũ����Һ���Ƶ����������ʵ����ʵ���n����Һ�����V����ģ�������ʱ���ؼ�Ҫ�����ƹ���������n��V�����ı仯����n������ֵС����V������ֵ��ʱ������ʹ������ҺŨ��ƫС����n������ֵ��V������ֵСʱ������ʹ������ҺŨ��ƫ��
��2����������100mL 0.9mol/L��ϡ����IJ���Ը�������������
��3����������һ�����ʵ���Ũ�ȵIJ���ѡ������
��4������c=
| n |
| V |
���
�⣺��1����9mol/L��Ũ����ϡ�ͳ�0.9mol/L��ϡ����100mL������n=c1V1=c2V2��֪����ҪŨ��������Ϊ��
=0.01L=10mL��
�ʴ�Ϊ��10��
��2������100mL 0.9mol/L��ϡ����IJ���Ϊ�����㡢��ȡ��ϡ�͡���ȴ����Һ��ϴ�ӡ����ݺ�ҡ�ȵȣ���ȷ�IJ���˳��Ϊ��ADECBHGF��
�ʴ�Ϊ��ADECBHGF��
��3����������100mL 0.9mol/L��ϡ����IJ����֪����Ҫʹ�õ������У���Ͳ���ձ�����������100mL����ƿ����ͷ�ιܵȣ�
�ʴ�Ϊ��100mL����ƿ������������Ͳ����ͷ�ιܣ�
��4��A��ʹ������ƿ������Һʱ������Һ�棬���������ˮ��������ƿ�̶��ߣ��������Ƶ���Һ���ƫС��������Һ��Ũ��ƫ��A��ȷ��
B��û��������ˮϴ�ձ�2-3�Σ�����ϴҺ��������ƿ�У��ᵼ�����Ƶ���Һ�����ʵ����ʵ���ƫС�����Ƶ���ҺŨ��ƫ�ͣ���B����
C������ƿ������ˮϴ����û�к�ɣ�����ƿ����Ҫ��ɣ������ʵ����ʵ�������Һ������������䣬��Ӱ�����ƽ������C����
D������ʱ���μ�����ˮ����ʹҺ���Ը��ڿ̶��ߣ��������Ƶ���Һ���ƫ�����Ƶ���ҺŨ��ƫ�ͣ���D����
E������õ���Һ����������ˮϴ����ĩ�ɵ��Լ�ƿ�б��ã��Լ�ƿ�е�����ˮ�����Ƶ���Һϡ�ͣ�������ҺŨ��ƫС����E����
�ʴ�Ϊ��A��
| 0.9mol/L��0.1L |
| 9mol/L |
�ʴ�Ϊ��10��
��2������100mL 0.9mol/L��ϡ����IJ���Ϊ�����㡢��ȡ��ϡ�͡���ȴ����Һ��ϴ�ӡ����ݺ�ҡ�ȵȣ���ȷ�IJ���˳��Ϊ��ADECBHGF��
�ʴ�Ϊ��ADECBHGF��
��3����������100mL 0.9mol/L��ϡ����IJ����֪����Ҫʹ�õ������У���Ͳ���ձ�����������100mL����ƿ����ͷ�ιܵȣ�
�ʴ�Ϊ��100mL����ƿ������������Ͳ����ͷ�ιܣ�
��4��A��ʹ������ƿ������Һʱ������Һ�棬���������ˮ��������ƿ�̶��ߣ��������Ƶ���Һ���ƫС��������Һ��Ũ��ƫ��A��ȷ��
B��û��������ˮϴ�ձ�2-3�Σ�����ϴҺ��������ƿ�У��ᵼ�����Ƶ���Һ�����ʵ����ʵ���ƫС�����Ƶ���ҺŨ��ƫ�ͣ���B����
C������ƿ������ˮϴ����û�к�ɣ�����ƿ����Ҫ��ɣ������ʵ����ʵ�������Һ������������䣬��Ӱ�����ƽ������C����
D������ʱ���μ�����ˮ����ʹҺ���Ը��ڿ̶��ߣ��������Ƶ���Һ���ƫ�����Ƶ���ҺŨ��ƫ�ͣ���D����
E������õ���Һ����������ˮϴ����ĩ�ɵ��Լ�ƿ�б��ã��Լ�ƿ�е�����ˮ�����Ƶ���Һϡ�ͣ�������ҺŨ��ƫС����E����
�ʴ�Ϊ��A��
���������⿼��������һ�����ʵ���Ũ�ȵ���Һ�ķ������Ѷ��еȣ������������У�ע������ԣ����ض�ѧ�������������ͽ��ⷽ����ָ����ѵ��������������ѧ������˼ά�������Ͻ��Ĺ淶ʵ�����������������ѵ�������������ע����ȷ�������ķ�����

��ϰ��ϵ�д�
�����Ŀ
���ܱ������е�һ����������巢����Ӧ��xA��g��+yB��g��?zC��g����ƽ��ʱ��ã�c��A��Ϊ0.5mol/L�������¶Ȳ��䣬���������ݻ�����Ϊԭ����2�����ﵽ��ƽ��ʱ���c��A��Ϊ0.22mol/L�������й��ж϶Ե��ǣ�������
| A��x+y��z |
| B����ѧ��Ӧ���ʽ���ԭ���ĸ��� |
| C��A��B��ת���ʼ�С |
| D����������ƽ��Ħ��������С |
��NAΪ�����ӵ�������ֵ������˵����ȷ���ǣ�������
| A�������£�1mol?L-1��NH4NO3��Һ�к��е�ԭ�ӵ���ĿΪ2NA |
| B�������£�100mL 1mol?L-1 AlCl3��Һ����������������0.1NA |
| C����״���£�2.24L NO2��ԭ������һ������0.3NA |
| D����2mol SO2��1mol O2��ϣ�������Ӧת�Ƶĵ�������һ����4NA |
��1.12g���ۼ���25mL2mol/L��FeCl3��Һ�У���ַ�Ӧ�������ǣ�������
| A����ǡ�ý�Fe3+ȫ����ԭ |
| B������Ӧ����Һ�е���KSCN��Һ�����Ժ�ɫ |
| C����Һ��Ϊdz��ɫ |
| D��Fe2+��Fe3+���ʵ���֮��Ϊ6��1 |
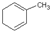 ��������Ȼ�̼��Һ��Ӧ���ɲ�������� �������������칹����������
��������Ȼ�̼��Һ��Ӧ���ɲ�������� �������������칹����������| A��1�� | B��2�� | C��3�� | D��4�� |
 ̼�͵��Ƕ�ֲ�����е���Ҫ���Ԫ�أ�������й����ŷŶ�����̼���������ЧӦ���������������⻯ѧ������Ŀǰ����Щ�ж��к�����Ĵ�����Ϊ��ѧ�о�����Ҫ���ݣ�
̼�͵��Ƕ�ֲ�����е���Ҫ���Ԫ�أ�������й����ŷŶ�����̼���������ЧӦ���������������⻯ѧ������Ŀǰ����Щ�ж��к�����Ĵ�����Ϊ��ѧ�о�����Ҫ���ݣ�