��Ŀ����
 ��1����д��ѧ����ʽ���ٱ���ϩ�Ӿ۷�Ӧ���ڱ�ϩ����ļӳɷ�Ӧ
��1����д��ѧ����ʽ���ٱ���ϩ�Ӿ۷�Ӧ���ڱ�ϩ����ļӳɷ�Ӧ��
��2��C4H9Cl��
��3����ͼ�л���ķ���ʽ��
��4��ij��A�ķ�����Ϊ84��Ӧ�ú�����ײ�֪�����к���̼̼˫����Ӧ�ú˴Ź������������ʾֻ��һ�����͵���ԭ�ӣ�д��A�ķ���ʽ
���㣺�л���Ľṹ������,�л���ʵ��ʽ�ͷ���ʽ��ȷ��,�л�������еĹ����ż���ṹ,ͬ���칹�����ͬ���칹��,���������������
ר�⣺�л���ѧ����
��������1���ٱ���ϩ��̼̼˫�����ѷ����Ӿ۷�Ӧ���ڱ�ϩ���巢���ӳɷ�Ӧ����1��2-������飻
��2��C4H10��ͬ���칹���У�CH3CH2CH2CH3��CH3CH��CH3��CH3�������仯ѧ������ͬ��Hԭ����Ŀ��������Clԭ���滻Hԭ�ӣ�
��3���ɽṹ��ʽ��֪����ʽ�������ţ�
��4��A������ͼ��������Է�������Ϊ84��������ױ��������к���̼̼˫������AӦΪϩ���������ʽΪCnH2n������14n=84��n=6������Ϊ�˴Ź������ױ���������ֻ��һ�����͵��⣬��ṹ��ʽӦΪ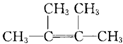 ��
��
��2��C4H10��ͬ���칹���У�CH3CH2CH2CH3��CH3CH��CH3��CH3�������仯ѧ������ͬ��Hԭ����Ŀ��������Clԭ���滻Hԭ�ӣ�
��3���ɽṹ��ʽ��֪����ʽ�������ţ�
��4��A������ͼ��������Է�������Ϊ84��������ױ��������к���̼̼˫������AӦΪϩ���������ʽΪCnH2n������14n=84��n=6������Ϊ�˴Ź������ױ���������ֻ��һ�����͵��⣬��ṹ��ʽӦΪ
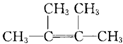 ��
�����
�⣺��1���ٱ���ϩ�Ӿ۷�ӦΪnC6H6-CH=CH2
 ���ʴ�Ϊ��nC6H6-CH=CH2
���ʴ�Ϊ��nC6H6-CH=CH2
 ��
��
�ڱ�ϩ����ˮ��Ӧ����1��2-������飬��Ӧ�Ļ�ѧ����ʽΪ��Br2+CH3CH2=CH2��BrCH2CH2BrCH3���ʴ�Ϊ��Br2+CH3CH2=CH2��BrCH2CH2BrCH3��
����ʽ�ԣ�
��2��C4H10��ͬ���칹���У�CH3CH2CH2CH3��CH3CH��CH3��CH3��
CH3CH2CH2CH3��������2�ֻ�ѧ������ͬ��Hԭ�ӣ���һ�ȴ�����2�ֱַ�Ϊ��CH3CH2CHClCH3��CH3CH2CH2CH2Cl��
CH3CH��CH3��CH3��������2�ֻ�ѧ������ͬ��Hԭ�ӣ���һ�ȴ�����2�ֱַ�Ϊ��CH3CCl��CH3��CH3��CH3CH��CH3��CH2Cl��
����4�֣���̼����ֻ����һ����-CH3����ͬ���칹��Ľṹ��ʽ��CH3CH2CH2CH2Cl��
�ʴ�Ϊ��4��CH3CH2CH2CH2Cl��
��3���ɽṹ��ʽ��֪����ʽΪC14H24O6�������й�����Ϊ�ǻ����Ȼ����ʴ�Ϊ��C14H24O6���ǻ����Ȼ���
��4��A������ͼ��������Է�������Ϊ84��������ױ��������к���̼̼˫������AӦΪϩ���������ʽΪCnH2n������14n=84��n=6������Ϊ�˴Ź������ױ���������ֻ��һ�����͵��⣬��ṹ��ʽӦΪ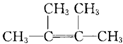 �������ʽΪC6H12���ṹ��ʽΪ��CH3��2C=C��CH3��2��̼̼˫��Ϊƽ��ṹ��������Cԭ�ӹ��棬������Ϊ2��3-����-2-��ϩ��A��ͬϵ���з�������С����Ϊ��ϩ����ṹ��ʽ��CH2=CH2��
�������ʽΪC6H12���ṹ��ʽΪ��CH3��2C=C��CH3��2��̼̼˫��Ϊƽ��ṹ��������Cԭ�ӹ��棬������Ϊ2��3-����-2-��ϩ��A��ͬϵ���з�������С����Ϊ��ϩ����ṹ��ʽ��CH2=CH2��
�ʴ�Ϊ��C6H12����CH3��2C=C��CH3��2���ǣ�2��3-����-2-��ϩ��CH2=CH2��
| ���� |
 ���ʴ�Ϊ��nC6H6-CH=CH2
���ʴ�Ϊ��nC6H6-CH=CH2| ���� |
 ��
���ڱ�ϩ����ˮ��Ӧ����1��2-������飬��Ӧ�Ļ�ѧ����ʽΪ��Br2+CH3CH2=CH2��BrCH2CH2BrCH3���ʴ�Ϊ��Br2+CH3CH2=CH2��BrCH2CH2BrCH3��
����ʽ�ԣ�
��2��C4H10��ͬ���칹���У�CH3CH2CH2CH3��CH3CH��CH3��CH3��
CH3CH2CH2CH3��������2�ֻ�ѧ������ͬ��Hԭ�ӣ���һ�ȴ�����2�ֱַ�Ϊ��CH3CH2CHClCH3��CH3CH2CH2CH2Cl��
CH3CH��CH3��CH3��������2�ֻ�ѧ������ͬ��Hԭ�ӣ���һ�ȴ�����2�ֱַ�Ϊ��CH3CCl��CH3��CH3��CH3CH��CH3��CH2Cl��
����4�֣���̼����ֻ����һ����-CH3����ͬ���칹��Ľṹ��ʽ��CH3CH2CH2CH2Cl��
�ʴ�Ϊ��4��CH3CH2CH2CH2Cl��
��3���ɽṹ��ʽ��֪����ʽΪC14H24O6�������й�����Ϊ�ǻ����Ȼ����ʴ�Ϊ��C14H24O6���ǻ����Ȼ���
��4��A������ͼ��������Է�������Ϊ84��������ױ��������к���̼̼˫������AӦΪϩ���������ʽΪCnH2n������14n=84��n=6������Ϊ�˴Ź������ױ���������ֻ��һ�����͵��⣬��ṹ��ʽӦΪ
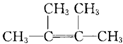 �������ʽΪC6H12���ṹ��ʽΪ��CH3��2C=C��CH3��2��̼̼˫��Ϊƽ��ṹ��������Cԭ�ӹ��棬������Ϊ2��3-����-2-��ϩ��A��ͬϵ���з�������С����Ϊ��ϩ����ṹ��ʽ��CH2=CH2��
�������ʽΪC6H12���ṹ��ʽΪ��CH3��2C=C��CH3��2��̼̼˫��Ϊƽ��ṹ��������Cԭ�ӹ��棬������Ϊ2��3-����-2-��ϩ��A��ͬϵ���з�������С����Ϊ��ϩ����ṹ��ʽ��CH2=CH2���ʴ�Ϊ��C6H12����CH3��2C=C��CH3��2���ǣ�2��3-����-2-��ϩ��CH2=CH2��
���������⿼���л���Ľṹ�����ʣ�Ϊ��Ƶ���㣬���չ����������ʵĹ�ϵΪ���Ĺؼ�������ϩ�����ʼ�ͬ���칹��Ŀ��飬��Ŀ�ѶȲ���4�����л����ƶ�Ϊ�����״��㣮

��ϰ��ϵ�д�
�����Ŀ
 ��ͼ��ʾʵ��װ�õ�K�պϣ������ж���ȷ���ǣ�������
��ͼ��ʾʵ��װ�õ�K�պϣ������ж���ȷ���ǣ�������| A��������Zn��a��b��Cu·������ |
| B��Ƭ�̺�ɹ۲쵽��ֽb����ɫ |
| C��Ƭ�̺�׳���c��SO42-������ |
| D��Cu�缫�Ϸ�����ԭ��Ӧ |
 һ���¶��£���ij�����̶����ܱ������м���������NH4��2S���壬�������·�Ӧ��
һ���¶��£���ij�����̶����ܱ������м���������NH4��2S���壬�������·�Ӧ�� ��д�����ķ���ϩ�ڴ����������ºϳɾ��ķ���ϩ�ķ�Ӧ����ʽ
��д�����ķ���ϩ�ڴ����������ºϳɾ��ķ���ϩ�ķ�Ӧ����ʽ