��Ŀ����
12��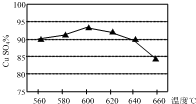 ��ij��ͭ����Ҫ�ɷ�ΪCuFeS2����O2��һ���¶ȷ�Χ�ڷ�����Ӧ����Ӧ���ù�������X�к���CuSO4��FeSO4��Fe2��SO4��3������SiO2�ȣ����Ӻ���Ƶô����ĵ������壨CuSO4•5H2O����
��ij��ͭ����Ҫ�ɷ�ΪCuFeS2����O2��һ���¶ȷ�Χ�ڷ�����Ӧ����Ӧ���ù�������X�к���CuSO4��FeSO4��Fe2��SO4��3������SiO2�ȣ����Ӻ���Ƶô����ĵ������壨CuSO4•5H2O������1��ʵ�����¶ȶԷ�Ӧ���ù���������ˮ����ͭ��CuSO4���ĺ�����Ӱ����ͼ��ʾ������������Ӧ���¶ȿ�����600�����ң��¶�������һ���̶Ⱥ�ˮ����ͭ�����½��Ŀ���ԭ����CuSO4�����˷ֽⷴӦ��
��2������г�����ؽ������������������������pH����ʼ������pH����������Ũ��Ϊ1.0mol•L-1���㣩��ʵ���п�ѡ�õ��Լ�����Ʒ��ϡ���ᡢ3% H2O2��Һ��CuO��������������pH��ֽ��
| ���� | Cu2+ | Fe2+ | Fe3+ |
| ��ʼ����ʱ��pH | 4.7 | 5.8 | 1.9 |
| ��ȫ����ʱ��pH | 6.7 | 9.0 | 3.2 |
�ڲ��������ɷ�Ӧ���ù�������X�Ƶô������������ʵ�鲽�裺
��һ�����������������ϡ���ᣬ���衢��ַ�Ӧ�����ˣ�
�ڶ���������Һ�м����Թ���3% H2O2��Һ����ַ�Ӧ��
������������Һ�м���CuO���þ���pH��ֽ����pH��3.2��4.7֮�䣬���ˣ�
���IJ�������Ũ������ȴ�ᾧ��
���岽�����ˡ�ϴ�ӣ����¸��
��3�������ԡ����������£�һ�ֽ�Thibacillus ferroxidans��ϸ���ܽ���ͭ��ת���������Σ��ù��̷�Ӧ�����ӷ���ʽΪ4CuFeS2+4H++17O2=4Cu2++4Fe3++8SO42-+2H2O��
���� ��1����ͼ��֪��600��ʱˮ����ͭ��CuSO4���ĺ�������ת�ۣ�ˮ����ͭ������ΪCuSO4•5H2O��������ͭ������ΪCuO���¶Ƚϸ�ʱ��CuSO4•5H2O�ɷֽ�����CuO��
��2��������ϡ����Һ���ʲ��������ʽ���㼴�ɣ�
�ڻ����X�к���CuSO4��FeSO4��Fe2��SO4��3������SiO2�ȣ������ܽ������ᣬ��ȥ�������裬Ȼ�����������������������ӣ��Ա㽵��pHֵ��ȥ����Ӧ������������������pHֵ��Ȼ�����Ũ������ȴ�ᾧ���ɣ�
��3�����ݷ�Ӧ����������������غ㶨����д��Ӧ�����ӷ�Ӧ����ʽ��
��� �⣺��1����ͼ��֪��600��ʱˮ����ͭ��CuSO4���ĺ�������ת�ۣ���Ӧ�����¶���600�����ң�ˮ����ͭ������ΪCuSO4•5H2O��������ͭ������ΪCuO���¶Ƚϸ�ʱ��CuSO4•5H2O�ɷֽ�����CuO������600������ʱˮ����ͭ�����ﺬ�����٣�
�ʴ�Ϊ��600�棻CuSO4�����˷ֽⷴӦ��
��2����ʵ��ʱ����Լ3%��H2O2��Һ100mL����������30%���ܶȽ���Ϊ1g•cm-3����H2O2�����ƣ���3%��100=30%��x����x=10mL������Ҫ30%��˫��ˮ10mL������ˮ90mL��
�ʲ���Ϊ������Ͳ��ȡ10mL30%H2O2��Һ�����ձ��У��ټ���90mL ˮ�����ˮϡ���� 100mL����������ȣ�
�ʴ�Ϊ������Ͳ��ȡ10mL30%H2O2��Һ�����ձ��У��ټ���90mL ˮ�����ˮϡ���� 100mL����������ȣ�
�ڻ����X�к���CuSO4��FeSO4��Fe2��SO4��3������SiO2�ȣ������ܽ������ᣬ��ȥ�������裬Ȼ�����������������������ӣ��Ա㽵��pHֵ��ȥ����Ӧ������������������pHֵ��Ȼ�����Ũ������ȴ�ᾧ��ϴ�ӡ����T�ɵò�Ʒ���ʲ����Ϊ������Һ�м����Թ���3% H2O2��Һ����ַ�Ӧ���Ա������������ӣ�
������Ϊ������Һ�м���CuO���þ���pH��ֽ����pH��3.2��4.7֮�䣬��������������������������ȥ��
������Ϊ������Ũ����
�ʴ�Ϊ������Һ�м����Թ���3% H2O2��Һ����ַ�Ӧ������Һ�м���CuO���þ���pH��ֽ����pH��3.2��4.7֮�䣻����Ũ����
��3����������Һ�������������Խ���ͭ�������������Σ���Ӧ������μӷ�Ӧ������������ͭ����������ˮ����Ӧ�ķ���ʽΪ4CuFeS2+2H2SO4+17O2=4CuSO4+2Fe2��SO4��3+2H2O�����ӷ�Ӧ����ʽΪ��4CuFeS2+4H++17O2=4Cu2++4Fe3++8SO42-+2H2O��
�ʴ�Ϊ��4CuFeS2+4H++17O2=4Cu2++4Fe3++8SO42-+2H2O��
���� ���⿼�鿼���Ϊ�ۺϣ��漰���ʵ��Ʊ������������ʵ����Ƶ����⣬�����ڿ���ѧ���ۺ����û�ѧ֪ʶ��������Ϊ�߿��������ͣ��Ѷ��еȣ�

 ��״Ԫ���źþ�ϵ�д�
��״Ԫ���źþ�ϵ�д�
| A�� | ʯī��O2����CO2�ķ�Ӧ�����ȷ�Ӧ | |
| B�� | ���ʯ��ʯī��O2��Ӧ���������ɾ��м��Թ��ۼ���CO2 | |
| C�� | �������Ƕȿ������ʯ��ʯī���ȶ� | |
| D�� | C�����ʯ��s���TC��ʯī��s����H=E3-E1 |
| A�� | Ԫ��Y��Z��W������ͬ���Ӳ�ṹ�����ӣ���뾶�������� | |
| B�� | 39g������Z2Y2��X2Y��Ӧת�Ƶ�����ԼΪ6.02��1023 | |
| C�� | Ԫ��Y��R�ֱ���Ԫ��X�γɵ�������������ȶ��ԣ�XmY��XmR | |
| D�� | Ԫ��Z��R���������ˮ�������Ӧ���ɵ��γ����Ի���� |
| A�� | ���� ����þ | B�� | ̼�� ̼���� | C�� | ʳ�� �ƾ� | D�� | ̼���� ���� |
| A�� | NH4+��Ba2+��NO3-��CO32- | B�� | Fe2+��OH-��SO42-��MnO4- | ||
| C�� | Na+��Mg2+��NO3-��SO42- | D�� | Na+��Fe3+��Cl-��AlO2- |
 ��ȡ�����������Ȫʵ��
��ȡ�����������Ȫʵ��