��Ŀ����
�����£���ijһԪ��HA��NaOH��Һ�������ϣ�������Һ��Ũ�Ⱥͻ�Ϻ�������Һ��pH���±���
��ش��������⣺��1��a 7���������������=������
��2���������������ʵ���������Ӷ������������д��HA���뷽��ʽ ��
��3�����ҡ��������������c1 0.2 ���������������=������
��4������ʵ�����û����Һ��������ʽ�ľ�ȷ��������������Ƽ��㣬�ش�ȷֵ�������һ��Ҫ����c��Na+��-c��A-��= mol/L��
| ʵ���� | HA���ʵ��� Ũ�ȣ�mol/L�� | NaOH���ʵ� ��Ũ�ȣ�mol/L�� | �����Һ��pH |
| �� | 0.2 | 0.2 | pH=a |
| �� | c1 | 0.2 | pH=7 |
| �� | 0.2 | 0.1 | pH��7 |
| �� | 0.1 | 0.1 | pH=9 |
��2���������������ʵ���������Ӷ������������д��HA���뷽��ʽ
��3�����ҡ��������������c1
��4������ʵ�����û����Һ��������ʽ�ľ�ȷ��������������Ƽ��㣬�ش�ȷֵ�������һ��Ҫ����c��Na+��-c��A-��=
���㣺��ѧƽ���Ӱ������
ר�⣺��ѧƽ��ר��
��������ʵ�鶡֪�������ʵ�������Ӧ����Һ�ʼ��ԣ�˵��HA�����ᣬ
��1���������ʵ�����ȣ���Ϊ�����ǿ�Ӧ����Ӧ����Һ�ʼ��ԣ�
��2������ĵ�����棬�ݴ���д����뷽��ʽ��
��3�������ǿ������ʵ�����Ӧʱ����Һ�Լ��ԣ��������ԣ�˵�����������
��4�������ʵ�������Ӧ����Һ�ʼ��ԣ���Һ������������ȫ�����������������ˮ��õ��ģ�c��OH-��=
��
��1���������ʵ�����ȣ���Ϊ�����ǿ�Ӧ����Ӧ����Һ�ʼ��ԣ�
��2������ĵ�����棬�ݴ���д����뷽��ʽ��
��3�������ǿ������ʵ�����Ӧʱ����Һ�Լ��ԣ��������ԣ�˵�����������
��4�������ʵ�������Ӧ����Һ�ʼ��ԣ���Һ������������ȫ�����������������ˮ��õ��ģ�c��OH-��=
| Kw |
| c(H+) |
���
�⣺��ʵ�鶡֪�������ʵ�������Ӧ����Һ�ʼ��ԣ�˵��HA�����ᣬ
��1��һԪ����HA��NaOH��Һ�������ϣ�������Һ�����ʵ���Ũ�ȶ�Ϊ0.2mol/L����Ӧ����Һ�ʼ��ԣ�pH��7��
�ʴ�Ϊ������
��2��HA�����ᣬ������棬����뷽��ʽΪ��HA?H++A-��
�ʴ�Ϊ��HA?H++A-��
��3��HA�����ᣬ��0.2mol/L��ǿ��NaOH�������Ϻ���Һ�����ԣ�˵�����������Ũ�ȴ��ڼ
�ʴ�Ϊ������
��4����Һ��pH=9����������Ũ��Ϊ10-9 mol/L�������ʵ�������Ӧ����Һ�ʼ��ԣ���Һ������������ȫ�����������������ˮ��õ��ģ�c��OH-��=
=
=10-5 mol/L��
һԪ��HA��NaOH��Һ������������NaA��Һ����Һ�д��ڵ���غ㣺c��Na+��+c��H+��=c��OH-��+c��A-����
������������õ���c��Na+��-c��A-��=c��OH-��-c��H+��=10-5-10-9��
�ʴ�Ϊ��10-5-10-9��
��1��һԪ����HA��NaOH��Һ�������ϣ�������Һ�����ʵ���Ũ�ȶ�Ϊ0.2mol/L����Ӧ����Һ�ʼ��ԣ�pH��7��
�ʴ�Ϊ������
��2��HA�����ᣬ������棬����뷽��ʽΪ��HA?H++A-��
�ʴ�Ϊ��HA?H++A-��
��3��HA�����ᣬ��0.2mol/L��ǿ��NaOH�������Ϻ���Һ�����ԣ�˵�����������Ũ�ȴ��ڼ
�ʴ�Ϊ������
��4����Һ��pH=9����������Ũ��Ϊ10-9 mol/L�������ʵ�������Ӧ����Һ�ʼ��ԣ���Һ������������ȫ�����������������ˮ��õ��ģ�c��OH-��=
| Kw |
| c(H+) |
| 10-14 |
| 10-9 |
һԪ��HA��NaOH��Һ������������NaA��Һ����Һ�д��ڵ���غ㣺c��Na+��+c��H+��=c��OH-��+c��A-����
������������õ���c��Na+��-c��A-��=c��OH-��-c��H+��=10-5-10-9��
�ʴ�Ϊ��10-5-10-9��
���������⿼������ϵ��жϣ���Ŀ�Ѷ��еȣ�����ע������������ݣ���������ʵĵ��������ˮ��ĽǶȽ��ѧϰ��ע����ع��ɺͷ����Ļ��ۣ�

��ϰ��ϵ�д�
�����Ŀ
���л�Ϊͬλ�ص��ǣ�������
| A��T��D |
| B��19K��20Ca |
| C�����ʯ��ʯī |
| D������̬���뻯��̬�� |
 ��ͼ��ѧУʵ���Ҵӻ�ѧ�Լ��̵���ص������Լ���ǩ�ϵIJ������ݣ��ݴˣ�����������⣺
��ͼ��ѧУʵ���Ҵӻ�ѧ�Լ��̵���ص������Լ���ǩ�ϵIJ������ݣ��ݴˣ�����������⣺ �����£���0.10mol?L-1NaOH��Һ�ζ� 20.00mL 0.10mol?L-1 CH3COOH��Һ�ζ�������ͼ��
�����£���0.10mol?L-1NaOH��Һ�ζ� 20.00mL 0.10mol?L-1 CH3COOH��Һ�ζ�������ͼ��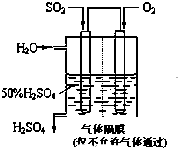 ���ô�������Ӧ��SO2ת��ΪSO3�ǹ�ҵ����������Ĺؼ����裮��֪��
���ô�������Ӧ��SO2ת��ΪSO3�ǹ�ҵ����������Ĺؼ����裮��֪��