��Ŀ����
13�� ��100mL��NaOH��Һ��ͨ��CO2��ַ�Ӧ���ڼ�ѹ�ͽϵ��¶��£�С�ĵؽ���Һ���ɣ��õ���ɫ����M��ͨ��CO2���������״������M������W�Ĺ�ϵ��ͼ��ʾ���Իش��������⣺
��100mL��NaOH��Һ��ͨ��CO2��ַ�Ӧ���ڼ�ѹ�ͽϵ��¶��£�С�ĵؽ���Һ���ɣ��õ���ɫ����M��ͨ��CO2���������״������M������W�Ĺ�ϵ��ͼ��ʾ���Իش��������⣺��1����NaOH��Һ�����ʵ���Ũ��Ϊ1mol/L��
��2��A��ʱ����ɫ����M�Ļ�ѧʽΪNa2CO3��ͨ��CO2�����Ϊ1120mL������״���£���
��3��B��ʱ����ɫ����M�Ļ�ѧʽΪNa2CO3��NaHCO3����ʹb�����ɵ��ε�������Ϊ8.4g��Ӧ��������Һ��ͨ�������̼1.792L����״���£���
��4���������ɵ�7.16g�ε���Һ�м���һ������NaOH����ַ�Ӧ��ѹ���������õ�������̼���ƹ��壨�ᾧˮ��8.4g���������������Ƶ����ʵ�����0.06mol��
���� ��ͼ֪NaOH����Ϊ4 g�����ʵ���Ϊ0.1 mol������c=$\frac{n}{V}$���㣻��ȫת��ΪNa2CO3ʱ��Na2CO3����Ϊ0.1mol��$\frac{1}{2}$��106g/mol=5.3 g����ȫת��ΪNaHCO3ʱ��NaHCO3����Ϊ0.1mol��84g/mol=8.4 g����A���ɫ����MΪNa2CO3��C���ɫ����MΪNaHCO3������̼ԭ���غ�ɵ�n��CO2��������V=nVm���������̼�����
ͼB��ʱM������Ϊ7.16 g��5.3��7.16��8.4��֪M��Na2CO3��NaHCO3��ɣ�����B��ʱNa2CO3���ʵ���Ϊx��NaHCO3���ʵ���Ϊy�������������غ㡢��������֮���з��̼���x��y��ֵ������V=nVm���������̼���������NaHCO3+NaOH=Na2CO3+H2O���㣮
��� �⣺��ͼ֪NaOH����Ϊ4 g�����ʵ���Ϊ0.1 mol����ȫת��ΪNa2CO3ʱ��Na2CO3����Ϊ0.1mol��$\frac{1}{2}$��106g/mol=5.3 g����ȫת��ΪNaHCO3ʱ��NaHCO3����Ϊ0.1mol��84g/mol=8.4 g����A���ɫ����MΪNa2CO3��C���ɫ����MΪNaHCO3��B��ʱ����ɫ����M�Ļ�ѧʽΪNa2CO3��NaHCO3��
��1����ͼ֪��������̼���Ϊ0ʱ�����������Ϊ4g����NaOH����Ϊ4 g�����ʵ���Ϊ0.1 mol����c=$\frac{n}{V}$=$\frac{0.1mol}{0.1L}$=1mol/L��
�ʴ�Ϊ��1mol/L��
��2��������������֪��A���ɫ����MΪNa2CO3����CO2���Ϊ0.1 mol��$\frac{1}{2}$��22.4L•mol-1=1.12L=1120 mL��
�ʴ�Ϊ��Na2CO3��1120��
��3��ͼB��ʱM������Ϊ7.16 g��5.3��7.16��8.4��֪M��Na2CO3��NaHCO3��ɣ�����B��ʱNa2CO3���ʵ���Ϊx��NaHCO3���ʵ���Ϊy��
��$\left\{\begin{array}{l}{106x+84y=7.16}\\{2x+y=0.1}\end{array}\right.$�����x=0.02��y=0.06��
��V��CO2��=��0.02 mol+0.06 mol����22.4L•mol-1=1.792L��
�ʴ�Ϊ��Na2CO3��NaHCO3��1.792��
��4���������ɵ�7.16g�ε���Һ�м���һ������NaOH����ַ�Ӧ��ѹ���������õ�������̼���ƹ��壨�ᾧˮ��8.4g��������ӦΪNaHCO3+NaOH=Na2CO3+H2O����֪NaHCO3���ʵ���Ϊ0.06mol���������������Ƶ����ʵ�����0.06mol��
�ʴ�Ϊ��0.06mol��
���� ���⿼���˻���ﷴӦ�ļ��㣬��Ŀ�Ѷ��еȣ���ȷ����Ԫ�ؼ��仯��������Ϊ���ؼ���ע����ȷ����ͼ�������߸����Ӧ������ɣ���������������ѧ���ķ������������������Ӧ��������

| A�� | O2��SO2 | B�� | NH3��N2 | C�� | NO��O2 | D�� | NH3��HCl |
| A�� | �����������ˮ��Ӧ�������ɼ��H2 | |
| B�� | ��������ʶ�������������ȵ������壬��ɫ��Ӧ�����ֻ�ɫ | |
| C�� | ��������ܶȶ�С��1g/cm3����˼�������ʶ����Ա�����ú���� | |
| D�� | ����������ڿ�����ȼ�ն����ɹ������� |
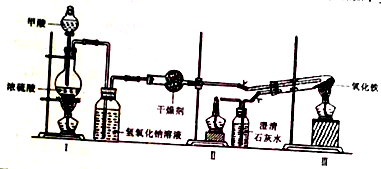
�ٰ�ͼ���Ӻ�װ�ã����װ�������ԣ�
�ڳ�ȡ0.2000gFe2O3��ʯӢ�Թ��У���ȼI���ƾ��ƣ���������״���
�������ij�������ȼ���������ƾ��ƣ�
��30min��ֹͣ���ȣ��رյ��ɼУ�
�ݴ�������ȴ�����º��ռ����
�������Ϸ����ֱ��ռ����������־ƾ��ƣ��������ֿ��Լ��л��桢����¶ȣ��;ƾ���Ƽ��ȵIJ��
��ش��������⣺
��1���Ʊ�CO��ԭ�������ü״���HCOOH����Ũ������������µķֽ��Ƶã�д���÷�Ӧ�Ļ�ѧ����ʽHCOOH $��_{��}^{Ũ����}$CO��+H2O��
��2��ʵ�鲽���Ӧ�ڼ���CO���Ⱥ��ȵ�ȼII���II����III�������ƾ��ƣ�
��3��ʵ�鲽��ݲ�����ȴ������ʱӦע�����ͨ��CO���������������
��4����֪FeO��Fe2O3��Fe3O4����Ԫ�ص����������ֱ�Ϊ��22.2%��30%��27.6%���������������3����Ʒ����Ԫ�������Ԫ�ص������������±���������Ԫ�ص�����������֪ǰ���ּ��ȷ�ʽ�õ��IJ���Ϊ�������оƾ��Ƽ������ò������������9�֣�
| ���ȷ�ʽ | ����Ԫ����� | ��Ԫ�ص���������/% | |
| Fe | O | ||
| �ƾ��� | Fe��O | 74.50 | 25.50 |
| �����־ƾ��� | Fe��O | 76.48 | 23.52 |
| �ƾ���� | Fe | 100.00 | 0.00 |
��6��ͨ�������ϻ�ȡ������Ϣ��I���ƾ���ƽ���¶�Ϊ600�棬�����־ƾ���ƽ���¶�Ϊ700�棬�ƾ���ƽ���¶�Ϊ930�森II������ָ������Ӧ�¶ȸ���710��Fe���ȶ����ڣ�680�桫710��֮�䣬FeO�ȶ����ڣ�����680�棬����Ҫ��Fe3O4���Է����ƾ��Ƽ�������������Fe��ԭ���dz�ʱ�伯�м���ʹ�ֲ��¶ȴﵽ��ԭ����������Ҫ���¶ȣ�����Fe�Ĺ����з��������з�Ӧ�Ļ�ѧ����ʽ3Fe2O3+CO$\frac{\underline{\;\;��\;\;}}{\;}$2Fe3O4+CO2��Fe3O4+CO$\frac{\underline{\;\;��\;\;}}{\;}$3FeO+CO2��Fe2O3+3CO$\frac{\underline{\;\;��\;\;}}{\;}$2Fe+3CO2��
 �����仯�������ճ����������Ӧ�ù㷺���о������仯�����Ӧ�������شش��������⣺
�����仯�������ճ����������Ӧ�ù㷺���о������仯�����Ӧ�������شش��������⣺��1����֪��¯���������лᷢ�����·�Ӧ��
FeO��s��+CO��g���TFe��s��+CO2��g����H1
Fe2O3��s��+$\frac{1}{3}$CO��g���T$\frac{2}{3}$Fe3O4��s��+$\frac{1}{3}$CO2��g����H2
Fe3O4��s��+4CO��g���T3Fe��s��+4CO2��g����H3
Fe2O3��s��+3CO��g���T2Fe��s��+3CO2��g����H4
���H4�ı���ʽΪ��H2+$\frac{2}{3}$��H3���ú���H1����H2����H3�Ĵ���ʽ��ʾ����
��2��������Ӧ�ڸ�¯�д��·�Ϊ�����Σ�������Ҫ�ɷ����¶ȵĹ�ϵ���£�
| �¶� | 250�� | 600�� | 1000�� | 2000�� |
| ��Ҫ�ɷ� | Fe2O3 | Fe3O4 | FeO | Fe |
m��Fe����m��O��=35��2����FeO��CO��ԭΪFe�İٷ���Ϊ80%�����������������в���Fe��OԪ�أ���
��3�����Ƚ���������CO��������Ӧ�Ĵ�������֪ij�ִ�������������ӦCO��g��+3H2��g��?CH4��g��+H2O��g����H��0����T�棬106Paʱ��l mol CO��3mol H2��������ɱ���ܱ������У�ʵ����CO���������x��CO�������
| t/min | 0 | 10 | 20 | 30 | 40 | 50 |
| x��CO�� | 0.25 | 0.23 | 0.214 | 0.202 | 0.193 | 0.193 |
a��������ѹǿ���ٷ����仯
b�����������ܶȲ��ٷ����仯
c��v����CO��=3v����H2��
d����������ƽ����Է����������ٷ����仯
�ڴﵽƽ��ʱCO��ת����Ϊ37.1%��
��ͼ��ʾ�÷�ӦCO��ƽ��ת�������¶ȡ�ѹǿ�Ĺ�ϵ��ͼ���¶�T1��T2��T3�ɸߵ��͵�˳����T3��T2��T1������������Ӧ���ȣ�����ͬѹǿ�£��¶Ƚ��ͣ�ƽ��������Ӧ�����ƶ���CO��ת����Խ�ߣ���
| A�� | ��ҽ����̼���ơ�Al��OH��3������������θ����� | |
| B�� | ���������۵�ܸߣ����������ͻ���ϣ�����Ҫ�ɷ���SiO2 | |
| C�� | ˮ��������������ճ�ϼ��ͷ���� | |
| D�� | ����ˮ�м�������������ˮ������Al��OH��3���������ɱ������������ |