��Ŀ����
����ѡ��������Ũ�ȹ�ϵ����ȷ���ǣ�������
| A��Na2CO3��Һ�У�c��OH-��=c��HCO3-��+2c��H2CO3��+c��H+�� |
| B��NaHCO3��Һ�У�c��Na+��=c��HCO3-��+c��CO32-��+c��H2CO3�� |
| C��pH=2��������Һ��pH=12�İ�ˮ���������Һ�У�cc��H+��+��NH4+��=c��OH-��+c��Cl-�� |
| D�������ʵ�����CH3COOH��CH3COONa��Һ��������������Һ�У�c��CH3COO-��+c��OH-��=c��H+��+c��CH3COOH�� |
���㣺����Ũ�ȴ�С�ıȽ�,����ˮ���Ӧ��
ר�⣺�����ˮ��ר��
������A�����������غ��жϣ�
B�����������غ��жϣ�
C�����õ���غ��жϣ�
D�������ʵ�����CH3COOH��CH3COONa��Һ�������ϣ���Һ�����ԣ�CH3COO-��ˮ��̶�С��CH3COOH�ĵ���̶ȣ���ϵ���غ��жϣ�
B�����������غ��жϣ�
C�����õ���غ��жϣ�
D�������ʵ�����CH3COOH��CH3COONa��Һ�������ϣ���Һ�����ԣ�CH3COO-��ˮ��̶�С��CH3COOH�ĵ���̶ȣ���ϵ���غ��жϣ�
���
�⣺A����̼������Һ�У����������غ㼴c��OH-��=c��HCO3-��+2c��H2CO3��+c��H+������A��ȷ��
B����̼��������Һ�У����������غ㼴c��Na+��=c��HCO3-��+c��CO32-��+c��H2CO3������B��ȷ��
C�������백ˮ�Ļ����Һ�У����ڵ���غ㣬��c��H+��+��NH4+��=c��OH-��+c��Cl-������C��ȷ��
D�������ʵ�����CH3COOH��CH3COONa��Һ�������ϣ���Һ�����ԣ�CH3COO-��ˮ��̶�С��CH3COOH�ĵ���̶ȣ���Һ�д��ڵ���غ㼴c��CH3COO-��+c��OH-��=c��H+��+c��Na+����c��Na+����c��CH3COOH������D����
��ѡD��
B����̼��������Һ�У����������غ㼴c��Na+��=c��HCO3-��+c��CO32-��+c��H2CO3������B��ȷ��
C�������백ˮ�Ļ����Һ�У����ڵ���غ㣬��c��H+��+��NH4+��=c��OH-��+c��Cl-������C��ȷ��
D�������ʵ�����CH3COOH��CH3COONa��Һ�������ϣ���Һ�����ԣ�CH3COO-��ˮ��̶�С��CH3COOH�ĵ���̶ȣ���Һ�д��ڵ���غ㼴c��CH3COO-��+c��OH-��=c��H+��+c��Na+����c��Na+����c��CH3COOH������D����
��ѡD��
���������⿼������Һ�еĵ���غ㡢�����غ㡢�����غ������غ㣬��Ŀ�Ѷ��еȣ�

��ϰ��ϵ�д�
 �»ƸԱ����ܾ�ϵ�д�
�»ƸԱ����ܾ�ϵ�д�
�����Ŀ
����ʵ��װ�������ȷ�����ܴﵽĿ���ǣ�������
A�� �ⶨ�Ҵ��Ľṹʽ |
B�� �ζ����ⶨ��������ʵ���Ũ�� |
C�� �ϳɰ������鰱������ |
D�� ������Ȼ�̼��Һ�з�����⣬���������Ȼ�̼ |
����ʱ����V1mL��c1mol?L-1�İ�ˮ�μӵ�V2mL��c2mol?L-1�������У���������һ����ȷ���ǣ�������
| A����c1=c2�����Һ��c��NH4+��=c��Cl-������V1��V2 |
| B���������Һ��pH=7����c1V1��c2V2 |
| C����V1��V2��c1=c2������Һ��pH��7 |
| D���������Һ��pH��7������Һ��c��NH4+����c��OH-����c��Cl-����c��H+�� |
����˵������ȷ���ǣ�������
| A������͵����ʶ��ܷ���ˮ�ⷴӦ |
| B��������͵�����Һ������Ũ������� |
| C���ױ���������һ�������¶��ܷ���ȡ����Ӧ |
| D��ֲ���ͺ�ʯ�͵��ѻ��������ʹ����KMnO4��Һ��ɫ |
�����Ƶ���ˮ�д���ƽ�⣺Cl2+H2O?HCl+HClO��������������������ʹc��HClO��������ǣ�������
| A��CaCO3 |
| B��CaSO3 |
| C��NaOH |
| D��HCl |
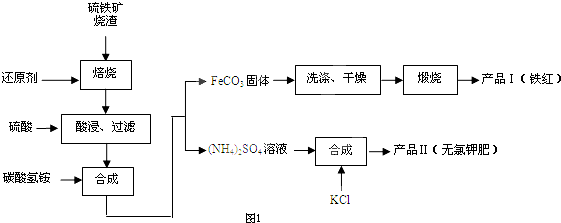
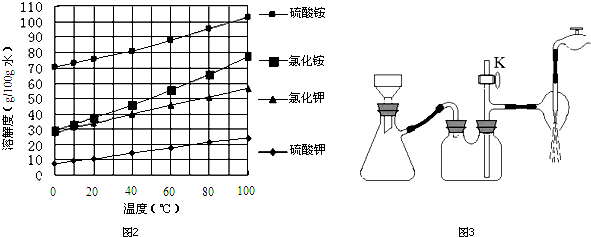
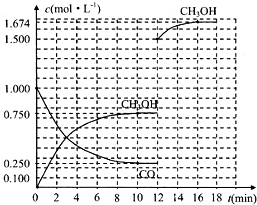
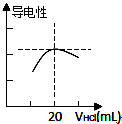 ijѧϰС�������кͷ�Ӧԭ���ⶨij��ˮ�����ʵ���Ũ�ȣ��Բ�����Һ�������жϵζ��յ㣮ʵ�鲽�����£�
ijѧϰС�������кͷ�Ӧԭ���ⶨij��ˮ�����ʵ���Ũ�ȣ��Բ�����Һ�������жϵζ��յ㣮ʵ�鲽�����£�