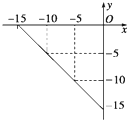
��Ŀ����
�����£�ȡ0.2mol/L HA��Һ��0.2mol/L NaOH��Һ�������ϣ���Ϻ���Һ����ı仯���ƣ�����û����Һ��pH=8���Իش��������⣺
��1����Ϻ����Һ����ˮ�������c��OH-�� 0.1mol/L NaOH��Һ����ˮ�������c��OH-�������������������=������
��2����֪NH4A��Һ�����ԣ���֪��HA��Һ�ӵ�NaHCO3��Һ��������ų������ƶ�NH4HCO3��Һ��pH 7�����������������=������
��3������ͬ�¶�����ͬŨ�ȵ���������Һ��A��NH4HCO3��B��NH4A��C����NH4��2SO4��D��NH4Cl����pH�ɴ�С��˳������ ������ĸ��ţ���
��1����Ϻ����Һ����ˮ�������c��OH-��
��2����֪NH4A��Һ�����ԣ���֪��HA��Һ�ӵ�NaHCO3��Һ��������ų������ƶ�NH4HCO3��Һ��pH
��3������ͬ�¶�����ͬŨ�ȵ���������Һ��A��NH4HCO3��B��NH4A��C����NH4��2SO4��D��NH4Cl����pH�ɴ�С��˳������
���㣺�����ʱ�Ķ����жϼ��й�ph�ļ���
ר�⣺����ƽ������Һ��pHר��
��������1�������������ӵ����ܴٽ�ˮ���룬����������ˮ���룻
��2����������֪��HA�����Ա�̼��ǿ��NH4A��ҺΪ���ԣ�˵��笠����Ӻ��������ˮ��̶���ͬ���ɴ˵�֪笠�����ˮ��̶�С��̼��������ӵ�ˮ�⣬����NH4HCO3��Һ�ʼ��ԣ�
��3������笠����ӵ�ˮ��̶��ж���Һ����ԵĴ�С����ҺŨ��Խϡ���ε�ˮ��̶�Խ��
��2����������֪��HA�����Ա�̼��ǿ��NH4A��ҺΪ���ԣ�˵��笠����Ӻ��������ˮ��̶���ͬ���ɴ˵�֪笠�����ˮ��̶�С��̼��������ӵ�ˮ�⣬����NH4HCO3��Һ�ʼ��ԣ�
��3������笠����ӵ�ˮ��̶��ж���Һ����ԵĴ�С����ҺŨ��Խϡ���ε�ˮ��̶�Խ��
���
�⣺��1�����κ������������ܴٽ�ˮ���룬����������ǿ��������ˮ���룬���Ի����Һ����ˮ�������c��H+����0.1mol?L-1NaOH��Һ����ˮ�������c��H+����
�ʴ�Ϊ������
��2����HA��Һ�ӵ�NaHCO3��Һ��������ų���˵��HA�����Ա�̼���ǿ��NH4A��ҺΪ���ԣ�˵����ͬ�����£���ˮ��HA�ĵ���̶���ͬ������NH4HCO3��笠����ӵ�ˮ��̶�С��̼��������ӵ�ˮ��̶ȣ�������Һ��pH��7��
�ʴ�Ϊ������
��3����NH4��2SO4 ��NH4Cl��ǿ�������Σ�笠�����ˮ�����Һ�����ԣ���Һ��笠�����Ũ��Խ��ˮ��̶�ԽС����ˮ��ĸ����࣬�����Ȼ����Һ��pHֵ��������泥�
NH4A��Һ���������ӵ�ˮ��̶���ȣ�������Һ�����ԣ���Һ��pHֵ�����Ȼ�泥�
NH4HCO3��Һ��笠����ӵ�ˮ��̶�С��̼��������ӵ�ˮ��̶ȣ���Һ�ʼ��ԣ�������Һ��pHֵ���
����pH�ɴ�С��˳������Ϊ��ABDC��
�ʴ�Ϊ��ABDC��
�ʴ�Ϊ������
��2����HA��Һ�ӵ�NaHCO3��Һ��������ų���˵��HA�����Ա�̼���ǿ��NH4A��ҺΪ���ԣ�˵����ͬ�����£���ˮ��HA�ĵ���̶���ͬ������NH4HCO3��笠����ӵ�ˮ��̶�С��̼��������ӵ�ˮ��̶ȣ�������Һ��pH��7��
�ʴ�Ϊ������
��3����NH4��2SO4 ��NH4Cl��ǿ�������Σ�笠�����ˮ�����Һ�����ԣ���Һ��笠�����Ũ��Խ��ˮ��̶�ԽС����ˮ��ĸ����࣬�����Ȼ����Һ��pHֵ��������泥�
NH4A��Һ���������ӵ�ˮ��̶���ȣ�������Һ�����ԣ���Һ��pHֵ�����Ȼ�泥�
NH4HCO3��Һ��笠����ӵ�ˮ��̶�С��̼��������ӵ�ˮ��̶ȣ���Һ�ʼ��ԣ�������Һ��pHֵ���
����pH�ɴ�С��˳������Ϊ��ABDC��
�ʴ�Ϊ��ABDC��
���������⿼�����ε�ˮ��ԭ������Һ���������ҺpH�ļ��㣬��Ŀ�Ѷ��еȣ��ѵ����ж���ͬ�¶ȡ���ͬŨ�����Ȼ�狀��������ҺpHֵ��С�ıȽϣ���ҺԽϡ笠����ӵ�ˮ��̶�Խ��ˮ��ĸ����٣����Ȼ����Һ��pHֵ�����������Һ��pHֵ��

��ϰ��ϵ�д�
 ȫ�ſ��䵥Ԫ�����������ܸ�ϰϵ�д�
ȫ�ſ��䵥Ԫ�����������ܸ�ϰϵ�д�
�����Ŀ
����Һ��pH��4������Һ��pH��5�������Һ������Һ��c��OH-��֮��Ϊ��������
| A��4��5 | B��1��10 |
| C��10��1 | D��1��2 |
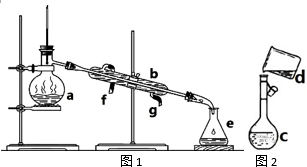 �����������ơ���װ��ʹ�÷�������ѧ��ѧʵ��Ļ�������ͼΪ����ʵ��װ�ã�
�����������ơ���װ��ʹ�÷�������ѧ��ѧʵ��Ļ�������ͼΪ����ʵ��װ�ã�