��Ŀ����
11����84����Һ������Чɱ�����H7N9������ijͬѧ���ġ�84����Һ��˵���е��䷽������NaClO�����Լ�����480mL��NaClO 25%���ܶ�Ϊ1.2g•cm-3������Һ������˵����ȷ���ǣ�������| A�� | ��Ҫ����NaClO���������Ϊ144.0 g | |
| B�� | ��ͼ��ʾ�������У��������Dz���Ҫ�ģ������һ�ֲ������� | |
| C�� | ���Ƶ���Һ�ڿ����й��գ����ú���Һ��NaClO�����ʵ���Ũ�ȼ�С | |
| D�� | ����ƿ������ˮϴ����Ӧ��ɲ���������Һ���ƣ�������ƫ�� |
���� A������ƿ�Ĺ��û��480mL��Ӧѡȡ500 mL������ƿ�������ƣ�
B����Ҫ���������衢������������Ҫ��ͷ�ιܣ�
C������NaClO�����տ����е�H2O��CO2�����ʣ�����NaClO����Һ���ʵ���NaClO���٣�
D������ƿ����ˮ������Һ�������Ӱ�죮
��� �⣺A��Ӧѡȡ500 mL������ƿ�������ƣ�Ȼ��ȡ��480mL���ɣ�������ҪNaClO��������500mL��1.2 g•cm-3��25%=150.0g����A����
B������������ƽ����NaClO���壬�����ձ����ܽ�NaClO�����ò��������н������������������ƿ�ͽ�ͷ�ι������ݣ�ͼʾ��A��B��C��D����Ҫ�������貣�����ͽ�ͷ�ιܣ���B����
C������NaClO�����տ����е�H2O��CO2�����ʣ�����NaClO����Һ���ʵ���NaClO���٣����Ƶ���Һ�����ʵ����ʵ�����С������Һ�����ʵ���Ũ��ƫС����C��ȷ��
D������ƿ����ˮ������Һ�������Ӱ�죬���Ծ�ϴ�Ӹɾ�������ƿ���غ�ɺ���ʹ�ã���D����
��ѡC��
���� ���⿼����һ�����ʵ���Ũ����Һ���ƵIJ��衢�����Լ��������ȣ��ѶȲ��Ǻܴ�ע��������ʵ�����ʱ����Һ�������500mL���㣮

��ϰ��ϵ�д�
�����Ŀ
1��ʵ���Ҳ��ü���װ��ģ�ҵ����ԭ����ʵ��װ����ͼ��ʵ�鲽�����£�
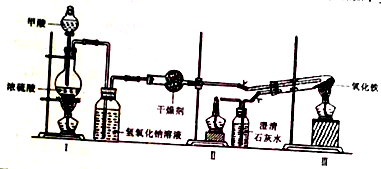
�ٰ�ͼ���Ӻ�װ�ã����װ�������ԣ�
�ڳ�ȡ0.2000gFe2O3��ʯӢ�Թ��У���ȼI���ƾ��ƣ���������״���
�������ij�������ȼ���������ƾ��ƣ�
��30min��ֹͣ���ȣ��رյ��ɼУ�
�ݴ�������ȴ�����º��ռ����
�������Ϸ����ֱ��ռ����������־ƾ��ƣ��������ֿ��Լ��л��桢����¶ȣ��;ƾ���Ƽ��ȵIJ��
��ش��������⣺
��1���Ʊ�CO��ԭ�������ü״���HCOOH����Ũ������������µķֽ��Ƶã�д���÷�Ӧ�Ļ�ѧ����ʽHCOOH $��_{��}^{Ũ����}$CO��+H2O��
��2��ʵ�鲽���Ӧ�ڼ���CO���Ⱥ��ȵ�ȼII���II����III�������ƾ��ƣ�
��3��ʵ�鲽��ݲ�����ȴ������ʱӦע�����ͨ��CO���������������
��4����֪FeO��Fe2O3��Fe3O4����Ԫ�ص����������ֱ�Ϊ��22.2%��30%��27.6%���������������3����Ʒ����Ԫ�������Ԫ�ص������������±���������Ԫ�ص�����������֪ǰ���ּ��ȷ�ʽ�õ��IJ���Ϊ�������оƾ��Ƽ������ò������������9�֣�
��5��ͨ����һ���������������ǰ���ּ��ȷ�ʽ�õ��Ĺ����ĩ�ɷ־�ΪFe3O4��Fe���þƾ���Ƽ��ȵõ��Ĺ����ĩ�ɷ�ΪFe����������þƾ��Ƽ��ȷ�ʽ�������Fe3O4��Fe��������Ϊ12��1��Ҫ������������
��6��ͨ�������ϻ�ȡ������Ϣ��I���ƾ���ƽ���¶�Ϊ600�棬�����־ƾ���ƽ���¶�Ϊ700�棬�ƾ���ƽ���¶�Ϊ930�森II������ָ������Ӧ�¶ȸ���710��Fe���ȶ����ڣ�680�桫710��֮�䣬FeO�ȶ����ڣ�����680�棬����Ҫ��Fe3O4���Է����ƾ��Ƽ�������������Fe��ԭ���dz�ʱ�伯�м���ʹ�ֲ��¶ȴﵽ��ԭ����������Ҫ���¶ȣ�����Fe�Ĺ����з��������з�Ӧ�Ļ�ѧ����ʽ3Fe2O3+CO$\frac{\underline{\;\;��\;\;}}{\;}$2Fe3O4+CO2��Fe3O4+CO$\frac{\underline{\;\;��\;\;}}{\;}$3FeO+CO2��Fe2O3+3CO$\frac{\underline{\;\;��\;\;}}{\;}$2Fe+3CO2��

�ٰ�ͼ���Ӻ�װ�ã����װ�������ԣ�
�ڳ�ȡ0.2000gFe2O3��ʯӢ�Թ��У���ȼI���ƾ��ƣ���������״���
�������ij�������ȼ���������ƾ��ƣ�
��30min��ֹͣ���ȣ��رյ��ɼУ�
�ݴ�������ȴ�����º��ռ����
�������Ϸ����ֱ��ռ����������־ƾ��ƣ��������ֿ��Լ��л��桢����¶ȣ��;ƾ���Ƽ��ȵIJ��
��ش��������⣺
��1���Ʊ�CO��ԭ�������ü״���HCOOH����Ũ������������µķֽ��Ƶã�д���÷�Ӧ�Ļ�ѧ����ʽHCOOH $��_{��}^{Ũ����}$CO��+H2O��
��2��ʵ�鲽���Ӧ�ڼ���CO���Ⱥ��ȵ�ȼII���II����III�������ƾ��ƣ�
��3��ʵ�鲽��ݲ�����ȴ������ʱӦע�����ͨ��CO���������������
��4����֪FeO��Fe2O3��Fe3O4����Ԫ�ص����������ֱ�Ϊ��22.2%��30%��27.6%���������������3����Ʒ����Ԫ�������Ԫ�ص������������±���������Ԫ�ص�����������֪ǰ���ּ��ȷ�ʽ�õ��IJ���Ϊ�������оƾ��Ƽ������ò������������9�֣�
| ���ȷ�ʽ | ����Ԫ����� | ��Ԫ�ص���������/% | |
| Fe | O | ||
| �ƾ��� | Fe��O | 74.50 | 25.50 |
| �����־ƾ��� | Fe��O | 76.48 | 23.52 |
| �ƾ���� | Fe | 100.00 | 0.00 |
��6��ͨ�������ϻ�ȡ������Ϣ��I���ƾ���ƽ���¶�Ϊ600�棬�����־ƾ���ƽ���¶�Ϊ700�棬�ƾ���ƽ���¶�Ϊ930�森II������ָ������Ӧ�¶ȸ���710��Fe���ȶ����ڣ�680�桫710��֮�䣬FeO�ȶ����ڣ�����680�棬����Ҫ��Fe3O4���Է����ƾ��Ƽ�������������Fe��ԭ���dz�ʱ�伯�м���ʹ�ֲ��¶ȴﵽ��ԭ����������Ҫ���¶ȣ�����Fe�Ĺ����з��������з�Ӧ�Ļ�ѧ����ʽ3Fe2O3+CO$\frac{\underline{\;\;��\;\;}}{\;}$2Fe3O4+CO2��Fe3O4+CO$\frac{\underline{\;\;��\;\;}}{\;}$3FeO+CO2��Fe2O3+3CO$\frac{\underline{\;\;��\;\;}}{\;}$2Fe+3CO2��
2�����й������ʵ���;��ȷ���ǣ�������
| A�� | ��ҽ����̼���ơ�Al��OH��3������������θ����� | |
| B�� | ���������۵�ܸߣ����������ͻ���ϣ�����Ҫ�ɷ���SiO2 | |
| C�� | ˮ��������������ճ�ϼ��ͷ���� | |
| D�� | ����ˮ�м�������������ˮ������Al��OH��3���������ɱ������������ |
1�����������У�������ֶ����ЧӦ�ķ�ɢϵ�ǣ�������
���������������ˮ�۶�����������Һ��FeCl3��Һ���ơ�����
���������������ˮ�۶�����������Һ��FeCl3��Һ���ơ�����
| A�� | �ڢܢ� | B�� | �ۢ� | C�� | �ڢܢ� | D�� | �ܢ٢� |