��Ŀ����
1����úΪ��Ҫԭ�Ͽ����Ʊ��Ҷ�������ع����������£�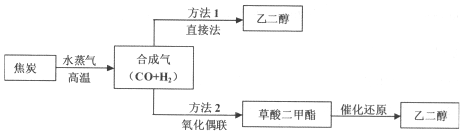
��1��д������l�ڴ�����������ֱ����ȡ�Ҷ����Ļ�ѧ����ʽ2CO+3H2$\frac{\underline{\;����\;}}{\;}$HOCH2CH2OH
��2���ϳ����ڲ�ͬ���������£����Ժϳɲ�ͬ�����ʣ��������ʽ��úϳ���Ϊԭ�Ͼ��ܵõ���ԭ��������Ϊ100%����B������ĸ����
A�����ᣨ HOOC-COOH�� B���״���CH3OH�� C������[CO��NH2��2]
��3����ҵ�ϻ�����������Ȼ������Ҫ�ɷ�ΪCH4������CO2��Ӧ�Ʊ��ϳ�������֪��
CH4��g��+2O2��g��=CO2��g��+2H2O��l����H=-890.3kJ/mol
2H2��g��+O2��g��=2H2O��l����H=-571.6kJ/mol
2CO��g��+O2��g��=2CO2��g����H=-566.0kJ/mol
��CH4��CO2���ɺϳ������Ȼ�ѧ����ʽΪCH4��g��+CO2��g��=2CO��g��+2H2��g����H=+247.3KJ/mol
��4������2���ں����ܱ�������Ͷ������������H2�������·�Ӧ��
CH3OOC-COOCH3��g��+4H2��g��?HOCH2CH2OH��g��+2CH3OH��g����H=-34kJ/mol
Ϊ����Ҷ����IJ��������ʣ��˲��õĴ�ʩ��BC������ĸ����
A�������¶� B������ѹǿ C����������Ũ��
��5�����������ˮ�����ɲ��CH3OOC-COOCH3+2H2O?2CH3OH+HOOC-COOH
�ٲ����Ƕ�Ԫ���ᣬ�����Ʊ�KHC2O4 ��������أ���KHC2O4 ��Һ�����ԣ��û�ѧƽ��ԭ�����ͣ�HC2O4-����ˮ�����ܵ��룺HC2O4-?H++C2O42-��HC2O4-+H2O?H2C2O4+OH-����HC2O4-�ĵ��������ˮ�⣮
����һ����KHC2O4 ��Һ�еμ�NaOH��Һ�����ԣ����й�ϵһ������ȷ����AC������ĸ����
A��c��Na+����c��K+����c��C2O42-����c��HC2O4-��
B��c��K+��=c��HC2O4-��+c��C2O42-��+c��H2C2O4��
C��c��K+��+c��Na+��=c��HC2O4-��+c��C2O42-��
��6���Ҷ�����������KOH��Һ�й���ȼ�ϵ�أ������Ҷ����ĵ缫Ϊ��Դ�ĸ������������������������ӦʽΪHOCH2CH2OH-10e-+14OH-=2CO32-+10H2O��
���� ����̼��ˮ������Ӧ�Ƶ�CO��H2��������CO��H2ֱ�ӷ�ͨ�����ȼӳɵõ��Ҷ�������������ż�����Ƶò������������������ͨ������ԭ�õ��Ҷ�����
��1�����ݷ�Ӧ����������Ϸ�Ӧ�ص���д��ѧ����ʽ��
��2�����������غ㶨����������
��3�����ݸ�˹������������
��4��ͨ������ƽ����ƶ����жϣ�
��5����HC2O4-����ˮ�����ܵ��룬�ݴ˷�����
����һ����KHC2O4��Һ�еμ�NaOH��Һ�����ԣ�����Һ�е�����Ϊ��KHC2O4��K2C2O4����������������غ�͵���غ���������
��6����ȼ�ϵ���У���ȼ�������������Ӧ�����ڸ����ŵ磬��ϵ������Һ�Ļ�������д������Ӧ��
��� �⣺��1���ϳ�������Ҫ�ɷ�ΪCO��H2���ڴ��������ºϳ��Ҷ����ķ�Ӧ��2CO+3H2$\frac{\underline{\;����\;}}{\;}$HOCH2CH2OH���ʴ�Ϊ��2CO+3H2$\frac{\underline{\;����\;}}{\;}$HOCH2CH2OH��
��2�����������غ㶨�ɿ�֪���ϳ�������Ҫ�ɷ�ΪCO��H2��������Ԫ�أ��ʲ����ܺϳɳ����أ���C��ѡ������A�Ҷ����У�C��Oԭ�Ӹ�����Ϊ1��2��������CO�е�1��1���ʲ����ɺϳ������ϳɣ���B���״���CH3OH�� ������CO��H2��1��2���ϳɣ�ȫ��ԭ�Ӿ�ת��ΪĿ����ԭ��ת���ʴﵽ��100%���ʴ�Ϊ��B��
��3����֪��CH4��g��+2O2��g���TCO2��g��+2H2O��l����H=-890.3kJ/mol ��
2H2��g��+O2��g���T2H2O��l����H=-571.6kJ/mol ��
2CO��g��+O2��g���T2CO2��g����H=-566��kJ/mol ��
����-��-�ۿɵã�
CH4��g��+CO2��g��=2CO��g��+2H2��g����H=��-890.3kJ/mol��-��-571.6kJ/mol��-��-566��kJ/mol��=+247.3KJ/mol���ʴ�Ϊ��CH4��g��+CO2��g��=2CO��g��+2H2��g����H=+247.3KJ/mol��
��4��A�������¶ȣ�ƽ�����ƣ��Ҷ����IJ������ͣ���A��ѡ��
B������ѹǿ����Ӧ���ʼӿ죬ƽ�����ƣ��Ҷ����IJ�������Bѡ��
C����������Ũ�ȣ���Ӧ���ʼӿ죬ƽ�����ƣ��Ҷ����IJ�������Cѡ���ʴ�Ϊ��BC��
��5����HC2O4-����ˮ�����ܵ��룺HC2O4-?H++C2O42-�����������ԣ�HC2O4-+H2O?H2C2O4+OH-��ˮ���Լ��ԣ���HHC2O4��Һ�����ԣ�˵��HC2O4-�ĵ��������ˮ�⣬�ʴ�Ϊ��HC2O4-����ˮ�����ܵ��룺HC2O4-?H++C2O42-��HC2O4-+H2O?H2C2O4+OH-����HC2O4-�ĵ��������ˮ�⣻
����һ����KHC2O4��Һ�еμ�NaOH��Һ�����ԣ�������NaOH����������٣�������KHC2O4��Ӧ��������Һ�е�����Ϊ��KHC2O4��K2C2O4��Na2C2O4��
A�����ڼ����NaOH����������٣�������KHC2O4��Ӧ������c��Na+�������ܴ���c��K+������A����
B����Һ�е�K+��HC2O4-��H2C2O4��C2O42-��������KHC2O4�����������غ��֪��c��K+��=c��HC2O4-��+c��C2O42-��+c��H2C2O4������B��ȷ��
C�����ݵ���غ��֪��c��K+��+c��Na+��=c��HC2O4-��+2c��C2O42-��+c��OH-������C����
�ʴ�Ϊ��AC��
��6����ȼ�ϵ���У���ȼ�������������Ӧ�����ڸ����ŵ磻�����Ǽ���ȼ�ϵ�أ��ʸ����Ҷ����ŵ�����CO32-���缫����ʽΪHOCH2CH2OH-10e-+14OH-=2CO32-+10H2O���ʴ�Ϊ������HOCH2CH2OH-10e-+14OH-=2CO32-+10H2O��
���� ���⿼���˸�˹���ɵ�Ӧ�á�ƽ����ƶ���ȼ�ϵ�ص缫��Ӧ����д�����ݣ��ۺ��Խ�ǿ���Ѷ����У�

NaBr+H2SO4�THBr+NaHSO4 ��
R-OH+HBr?R-Br+H2O ��
���ܴ��ڵĸ���Ӧ�У�����Ũ����Ĵ�������ˮ����ϩ���ѣ�Br-��Ũ��������ΪBr2�ȣ��й������б����£�
| �Ҵ� | ������ | ������ | 1-�嶡�� | |
| �ܶ�/g•cm-3 | 0.7893 | 1.4604 | 0.8098 | 1.2758 |
| �е�/�� | 78.5 | 38.4 | 117.2 | 101.6 |
��1���������1-�嶡����Ʊ�ʵ���У���������������õ�����d��������ĸ��
a��Բ����ƿ b����Ͳ c����ƿ d��������
��2���������ˮ���Դ��ڣ�����ڡ��������ڡ���С�ڡ�������
��3����1-�嶡��ֲ�Ʒ���ڷ�Һ©���м�ˮ�����ã��������²㣨��ϲ㡱�����²㡱���ֲ㡱����
��4���Ʊ������У������Ũ����������ϡ�ͣ���Ŀ����abc��������ĸ��
a�����ٸ�����ϩ���ѵ����� b������Br2������
c������HBr�Ļӷ� d��ˮ�Ƿ�Ӧ�Ĵ���
��5������ȥ������е���������Br2���������������ʺϵ���c��������ĸ��
a��NaI b��NaOH c��NaHSO3 d��KCl
��6�����Ʊ�������ʱ�����ñ߷�Ӧ����������ķ�������������ƽ��������������ķ����ƶ��������Ʊ�1-�嶡��ʱȴ���ܱ߷�Ӧ�����������ԭ����1-�嶡����������ķе�����
| A�� | Na+��K+��CO32-��NO3- | B�� | Mg2+��NH4+��SO42-��Cl- | ||
| C�� | Fe3+��K+��NO3-��Cl- | D�� | Ba2+��HCO3-��NO3-��K+ |
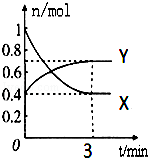 ��һ���¶��£����Ϊ2L���ܱ������У�NO2��N2O4֮�䷢����Ӧ��2NO2��g��������ɫ��?N2O4g������ɫ������ͼ��ʾ��
��һ���¶��£����Ϊ2L���ܱ������У�NO2��N2O4֮�䷢����Ӧ��2NO2��g��������ɫ��?N2O4g������ɫ������ͼ��ʾ��
 ��
�� NH3•H2O
NH3•H2O  NH4++OH-��
NH4++OH-�� ��
��