��Ŀ����
�±��г���ij�������ŷŵķ�ˮ�и��ɷݵĺ��������һ�����ֵ���й����ݣ�
ij���л���Ϊ�ó�������������������·�ˮ����ϵͳ��������Լ������ҵ��ˮ�����Ҵ���������ɱ���

��ش��������⣺
��1�����кͷ�Ӧ����ͨ��ѹ�������������� ��
��2������ˮ��ϸ�������ϸߣ�������Ư�۴���ʯ�������Zn2+��Ư�۳���Zn2+�����ӷ���ʽ�� ��
��3�����ദ��ϵͳ��ȥ����ɵõ��Ĺ�ҵ����Ʒ�� ���ѧʽ����
��4����ĤҺ��������ǽ�������ķ���ϩר��������ȫ�Զ�����ϵͳ�����ؽ����һ��Ĺ�Һ�����豸��Ϊ��߱�ĤҺ��������Ĺ�����������ˮ����ϵͳ����һ��ʱ�����Ҫ�Ա�Ĥ���л�ѧ��������Ĥ���ڴ����ķ����� ��
��5�����������մ�����ķ�ˮpH=8����ʱ��ˮ��Zn2+��Ũ��Ϊ mg/L�������£�Ksp[Zn��OH��2]=1.2��10-17���� ������ϡ������ϡ������һ�������
| ������п��ˮˮ�� | ���������ˮ���һ�����ֵ | |
| Zn2+Ũ��/��mg?L-1�� | ��800 | ��3.9 |
| pH | 1��5 | 6��9 |
| SO42-Ũ��/��mg?L-1�� | ��23 000 | ��150 |

��ش��������⣺
��1�����кͷ�Ӧ����ͨ��ѹ��������������
��2������ˮ��ϸ�������ϸߣ�������Ư�۴���ʯ�������Zn2+��Ư�۳���Zn2+�����ӷ���ʽ��
��3�����ദ��ϵͳ��ȥ����ɵõ��Ĺ�ҵ����Ʒ��
��4����ĤҺ��������ǽ�������ķ���ϩר��������ȫ�Զ�����ϵͳ�����ؽ����һ��Ĺ�Һ�����豸��Ϊ��߱�ĤҺ��������Ĺ�����������ˮ����ϵͳ����һ��ʱ�����Ҫ�Ա�Ĥ���л�ѧ��������Ĥ���ڴ����ķ�����
��5�����������մ�����ķ�ˮpH=8����ʱ��ˮ��Zn2+��Ũ��Ϊ
���㣺���ʷ�����ᴿ�ķ����ͻ��������ۺ�Ӧ��
ר�⣺ʵ�������
��������ˮ�����кͷ�Ӧ�أ�����ʯ���飬ѹ���������Ƿ�Ӧ��ֽ��У��������ճ�ͨ����ĤҺ�����������õ������ռ��ؽ������ദ�����õ���Һ����HCl������PH���õ�ѭ��ʹ�õ�����
��1��ѹ�������Ƿ��ý��г��ף�
��2��Ư�ۺ�п����ˮ��Һ��˫ˮ������������п�����ʹ����
��3�����ദ��ϵͳ��ȥ����ɵõ��Ĺ�ҵ����Ʒ��������п������ƣ�
��4����Ĥ���л�ѧ��������������ݻָ���
��5�������ܶȻ��������㣬���ͼ�����ݷ����Ƿ���ϱ���
��1��ѹ�������Ƿ��ý��г��ף�
��2��Ư�ۺ�п����ˮ��Һ��˫ˮ������������п�����ʹ����
��3�����ദ��ϵͳ��ȥ����ɵõ��Ĺ�ҵ����Ʒ��������п������ƣ�
��4����Ĥ���л�ѧ��������������ݻָ���
��5�������ܶȻ��������㣬���ͼ�����ݷ����Ƿ���ϱ���
���
�⣺��1�����кͷ�Ӧ����ͨ��ѹ��������������ʹ��Ӧ��ֽ��У��ʴ�Ϊ��ʹ��Ӧ��ֽ��У�
��2������ˮ��ϸ�������ϸߣ�������Ư�۴���ʯ�������Zn2+��Ư�ۺ�п����ˮ��Һ��˫ˮ������������п�����ʹ����ᣬ��Ӧ�����ӷ���ʽΪ��2ClO-+Zn2++2H2O=2HClO+Zn��OH��2�����ʴ�Ϊ��2ClO-+Zn2++2H2O=2HClO+Zn��OH��2����
��3�����ݹ��̷�����֪���ദ��ϵͳ��ȥ����ɵõ��Ĺ�ҵ����Ʒ��CaSO4��Zn��OH��2 ���ʴ�Ϊ��CaSO4��Zn��OH��2 ��
��4��ĤҺ��������ǽ�������ķ���ϩר��������ȫ�Զ�����ϵͳ�����ؽ����һ��Ĺ�Һ�����豸��Ϊ��߱�ĤҺ��������Ĺ�����������ˮ����ϵͳ����һ��ʱ�����Ҫ�Ա�Ĥ���л�ѧ��������Ĥ���ڴ����ķ�������������ݱ�Ĥ��
�ʴ�Ϊ����������ݱ�Ĥ��
��5����ˮpH=8��c��H+��=10-8mol/L��c��OH-��=10-6mol/L��Ksp[Zn��OH��2]=c��Zn2+��c2��OH-��=1.2��10?-17 ��
c��Zn2+��=
=1.2��10-5mol/L����ʱ��ˮ��Zn2+��Ũ��Ϊ1.2��10-5mol/L=[1.2��10-6mol��65g/mol]/L=0.78mg/L�����Ϲ��һ�������
�ʴ�Ϊ��0.78�����ϣ�
��2������ˮ��ϸ�������ϸߣ�������Ư�۴���ʯ�������Zn2+��Ư�ۺ�п����ˮ��Һ��˫ˮ������������п�����ʹ����ᣬ��Ӧ�����ӷ���ʽΪ��2ClO-+Zn2++2H2O=2HClO+Zn��OH��2�����ʴ�Ϊ��2ClO-+Zn2++2H2O=2HClO+Zn��OH��2����
��3�����ݹ��̷�����֪���ദ��ϵͳ��ȥ����ɵõ��Ĺ�ҵ����Ʒ��CaSO4��Zn��OH��2 ���ʴ�Ϊ��CaSO4��Zn��OH��2 ��
��4��ĤҺ��������ǽ�������ķ���ϩר��������ȫ�Զ�����ϵͳ�����ؽ����һ��Ĺ�Һ�����豸��Ϊ��߱�ĤҺ��������Ĺ�����������ˮ����ϵͳ����һ��ʱ�����Ҫ�Ա�Ĥ���л�ѧ��������Ĥ���ڴ����ķ�������������ݱ�Ĥ��
�ʴ�Ϊ����������ݱ�Ĥ��
��5����ˮpH=8��c��H+��=10-8mol/L��c��OH-��=10-6mol/L��Ksp[Zn��OH��2]=c��Zn2+��c2��OH-��=1.2��10?-17 ��
c��Zn2+��=
| 1.2��10-17 |
| (10-6)2 |
�ʴ�Ϊ��0.78�����ϣ�
���������⿼�������ʷ��뷽����ʵ��������ܶȻ�������������жϣ����ջ����ǹؼ�����Ŀ�Ѷ��еȣ�

��ϰ��ϵ�д�
 ��ս�п�����ϵ�д�
��ս�п�����ϵ�д�
�����Ŀ
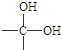 ��
��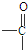 +H2O
+H2O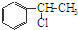 ��
��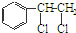 �ȶ���A��Cl2������Ӧ���ɵIJ��E��һ�ָ߷��ӻ���������ܺã�������һЩ������ǣ�������һЩС���Ӷ��Ѿ���ȥ��
�ȶ���A��Cl2������Ӧ���ɵIJ��E��һ�ָ߷��ӻ���������ܺã�������һЩ������ǣ�������һЩС���Ӷ��Ѿ���ȥ��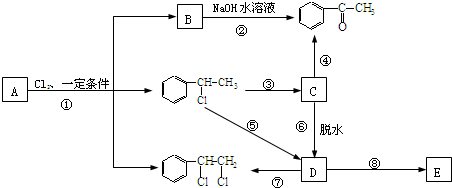

 ������������������
�����л����к����������У� ��ϵͳ������
��ϵͳ������
 ��R��R���ʾ�������ԭ�ӣ�
��R��R���ʾ�������ԭ�ӣ� ��
�� ��ǿ����������������
��ǿ���������������� ����һ���л���
����һ���л��� ��1�����ǵ�ѭ�������е���Ҫ���ʣ����ĺϳ���Ŀǰ�ձ�ʹ�õ��˹��̵�������
��1�����ǵ�ѭ�������е���Ҫ���ʣ����ĺϳ���Ŀǰ�ձ�ʹ�õ��˹��̵�������