��Ŀ����
����ʽ�ζ���ȷ��ȡ25.00mLijδ֪Ũ�ȵ���������һ�ྻ����ƿ�У�Ȼ����20mol?L-1������������Һ��ָʾ��Ϊ��̪�����ζ�������£�
��1�������������ݿ��Լ������������ʵ���Ũ��Ϊ mol?L-1��
��2���ﵽ�ζ��յ�ı�־��
��3�����²�����ɲⶨ���ƫ�ߵ�ԭ������� ��
A�����Ʊ���Һ�����������л���Na2CO3����
B���ζ��յ����ʱ�����ӵζ��ܵĿ̶ȣ�������������ȷ
C��ʢװδ֪Һ����ƿ������ˮϴ����δ�ô���Һ��ϴ
D��δ�ñ�Һ��ϴ��ʽ�ζ���
��4���ζ�ʱ�ߵα�ҡ����ƿ���۾�Ӧ�۲� ��
A���ζ�����Һ��ı仯?B����ƿ����Һ��ɫ�ı仯?
��5�����и����ʵ���Һ���ʵ���Ũ�Ⱦ�Ϊ0.1mol/L��
��HCl ��H2SO4 ��CH3COOH ������pH��С���������˳��Ϊ ��
| NaOH��ʼ���� | NaOH�յ���� | |
| ��һ�� | 0.10mL | 18.60mL |
| �ڶ��� | 0.30mL | 18.00mL |
��2���ﵽ�ζ��յ�ı�־��
��3�����²�����ɲⶨ���ƫ�ߵ�ԭ�������
A�����Ʊ���Һ�����������л���Na2CO3����
B���ζ��յ����ʱ�����ӵζ��ܵĿ̶ȣ�������������ȷ
C��ʢװδ֪Һ����ƿ������ˮϴ����δ�ô���Һ��ϴ
D��δ�ñ�Һ��ϴ��ʽ�ζ���
��4���ζ�ʱ�ߵα�ҡ����ƿ���۾�Ӧ�۲�
A���ζ�����Һ��ı仯?B����ƿ����Һ��ɫ�ı仯?
��5�����и����ʵ���Һ���ʵ���Ũ�Ⱦ�Ϊ0.1mol/L��
��HCl ��H2SO4 ��CH3COOH ������pH��С���������˳��Ϊ
���㣺�к͵ζ�
ר�⣺����ƽ������Һ��pHר��
��������1���ȼ������������������Һ��ƽ�������Ȼ�����c�����⣩=
�������������ʵ����Ѷȣ�
��2�����ݵζ��յ�ǰ��ҺΪ��ɫ���ζ��յ�ʱ��ҺΪ��ɫ�жϵζ��յ㣻
��3�����ݲ�����c�����⣩=
��Ӱ����з����ζ���
��4���ζ��������۾�Ӧ��ע����ƿ����Һ��ɫ�仯��
��5��ǿ����ȫ���������ȫ���룮
| c(��)��V(��) |
| V(����) |
��2�����ݵζ��յ�ǰ��ҺΪ��ɫ���ζ��յ�ʱ��ҺΪ��ɫ�жϵζ��յ㣻
��3�����ݲ�����c�����⣩=
| c(��)��V(��) |
| V(����) |
��4���ζ��������۾�Ӧ��ע����ƿ����Һ��ɫ�仯��
��5��ǿ����ȫ���������ȫ���룮
���
�⣺��1�����ݱ��������ݿ�֪����һ�εζ���������������Һ�����Ϊ����18.60-0.10��mL=18.50mL���ڶ��εζ������������Ƶ����Ϊ����18.00-0.30��mL=17.70mL�������������������ƽ��ֵΪ��
mL=18.10 mL��
c��NaOH��=
mol/L��27.62mol/L��
�ʴ�Ϊ��27.62��
��2���ζ�����֮ǰ����ҺΪ��ɫ���ζ�����ʱ��Һ��ɺ�ɫ�����Եζ��յ�����Ϊ�������һ����Һ����ɫ��dz��ɫ������Ӳ���ɫ��
�ʴ�Ϊ�������һ����Һ����ɫ��dz��ɫ������Ӳ���ɫ��
��3��A�����Ʊ���Һ�����������л���Na2CO3���ʣ�����V��NaOH��ƫ�ߣ���c�����⣩=
������c�����⣩ƫ�ͣ���A����
B���ζ��յ����ʱ�����ӵζ��ܵĿ̶ȣ�������������ȷ������V��NaOH��ƫ�ͣ���c�����⣩=
������c�����⣩ƫ�ߣ���B��ȷ��
C��ʢװδ֪Һ����ƿ������ˮϴ����δ�ô���Һ��ϴ��������ȷ���Խ����Ӱ�죬��C����
D��δ�ñ�Һ��ϴ��ʽ�ζ��ܣ�����V��NaOH��ƫ�ߣ���c�����⣩=
������c�����⣩ƫ�ͣ���D����
��ѡB��
��4���ζ������У��۾�Ӧ��ע����ƿ����Һ����ɫ�仯���Ա㼰ʱ�жϵζ��յ㣬
�ʴ�Ϊ��B��
��5��ǿ����ȫ���������ȫ���룬�����Ƕ�Ԫǿ�ᣬ������һԪǿ�ᣬ������һԪ���ᣬ��pH��С�����˳��Ϊ���ڢ٢ۣ��ʴ�Ϊ���ڢ٢ۣ�
| 18.50+17.70 |
| 2 |
c��NaOH��=
| 20��0.025 |
| 0.0181 |
�ʴ�Ϊ��27.62��
��2���ζ�����֮ǰ����ҺΪ��ɫ���ζ�����ʱ��Һ��ɺ�ɫ�����Եζ��յ�����Ϊ�������һ����Һ����ɫ��dz��ɫ������Ӳ���ɫ��
�ʴ�Ϊ�������һ����Һ����ɫ��dz��ɫ������Ӳ���ɫ��
��3��A�����Ʊ���Һ�����������л���Na2CO3���ʣ�����V��NaOH��ƫ�ߣ���c�����⣩=
| c(��)��V(��) |
| V(����) |
B���ζ��յ����ʱ�����ӵζ��ܵĿ̶ȣ�������������ȷ������V��NaOH��ƫ�ͣ���c�����⣩=
| c(��)��V(��) |
| V(����) |
C��ʢװδ֪Һ����ƿ������ˮϴ����δ�ô���Һ��ϴ��������ȷ���Խ����Ӱ�죬��C����
D��δ�ñ�Һ��ϴ��ʽ�ζ��ܣ�����V��NaOH��ƫ�ߣ���c�����⣩=
| c(��)��V(��) |
| V(����) |
��ѡB��
��4���ζ������У��۾�Ӧ��ע����ƿ����Һ����ɫ�仯���Ա㼰ʱ�жϵζ��յ㣬
�ʴ�Ϊ��B��
��5��ǿ����ȫ���������ȫ���룬�����Ƕ�Ԫǿ�ᣬ������һԪǿ�ᣬ������һԪ���ᣬ��pH��С�����˳��Ϊ���ڢ٢ۣ��ʴ�Ϊ���ڢ٢ۣ�
������������Ҫ����������к͵ζ�������������Ŀ�Ѷ��еȣ�ע������к͵ζ��IJ����������������ķ���������������ѧ���ķ������������������Ӧ����ѧ֪ʶ����������һ�����������⣮

��ϰ��ϵ�д�
�����Ŀ
��NAΪ�����ӵ�����������������ȷ���ǣ�������
| A��80gNH4NO3 �к��е�ԭ����Ϊ2NA |
| B��1Llmol?L-1�����к����Ȼ��������ΪNA |
| C����״���£�11.2LCCl4 ����������Ϊ0.5NA |
| D������������ȼ�գ�1lmolFeʧȥ�ĵ�����Ϊ2NA |
���бȽϣ�����ȷ���ǣ�������
| A�������ԣ�Na��Mg��Al |
| B��ԭ�Ӱ뾶��Na��S��O |
| C�����ԣ�KOH��NaOH��LiOH |
| D�����ԣ�HIO4��HBrO4��HClO4 |
�����Լ����ü���Na2CO3��Һ��NaHCO3��Һ���ǣ�������
| A������ | B������ʯ��ˮ |
| C��NaCl��Һ | D��NaOH��Һ |

 ��YΪ-OH��-OR��-X��±ԭ�ӣ���-NH2�ȣ������Լ����л��ϳ�����;�����һ���Լ������������ʻ��ӳɣ�������Ӧ��
��YΪ-OH��-OR��-X��±ԭ�ӣ���-NH2�ȣ������Լ����л��ϳ�����;�����һ���Լ������������ʻ��ӳɣ�������Ӧ��


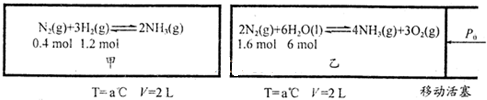
 �Ķ�����ʵ�����ݣ�������ĿҪ��ش����⣮
�Ķ�����ʵ�����ݣ�������ĿҪ��ش����⣮