��Ŀ����
18����NA��ʾ�����ӵ�������ֵ������˵����ȷ���ǣ�������| A�� | 1mol•L-1 Na2CO3��Һ�У���CO${\;}_{3}^{2-}$��ĿС��NA | |
| B�� | 1mol FeCl3��ȫת��Ϊ����������������н���������ĿΪNA | |
| C�� | 25��ʱ��1L pH=12��Ba��OH��2��Һ�к��е�OH-��ĿΪ0.02NA | |
| D�� | 1mol���ͱ�����Ļ������ȫȼ��ʱ����O2�ķ�����Ϊ7.5NA |
���� A����Һ�������ȷ��
B��һ���������������Ƕ�����������ľۼ��壻
C��pH=12������������Һ�У���������Ũ��Ϊ0.01mol/L��
D��1mol����1mol������ȼ��ʱ������7.5mol������
��� �⣺A����Һ�������ȷ������Һ�е�̼����ĸ��������㣬��A����
B��һ���������������Ƕ�����������ľۼ��壬��1mol�Ȼ����γɵ��������������ĸ���С��NA������B����
C��pH=12������������Һ�У���������Ũ��Ϊ0.01mol/L����1L�ĸ���Һ�е��������ĸ���Ϊ0.01NA������C����
D��1mol����1mol������ȼ��ʱ������7.5mol��������1mol���ͱ�����Ļ����ȼ������7.5mol��������7.5NA���������ӣ������ߵı����أ���D��ȷ��
��ѡD��
���� ���⿼���˰���٤���������йؼ��㣬�������ʵ����ļ��㹫ʽ�����ʽṹ�ǽ���ؼ����ѶȲ���

��ϰ��ϵ�д�
 ��ĩ1�����ʽ���������ϵ�д�
��ĩ1�����ʽ���������ϵ�д�
�����Ŀ
9�����й���һ���ͷ��������ǣ�������
| A�� | ���Ϸ�Ӧ | B�� | �ֽⷴӦ | ||
| C�� | ��Ӧ������л�ѧ������ | D�� | ԭ����ϳɷ��� |
6�����и�����������ԭ�Ӷ����������Ϊ8�����ȶ��ṹ���ǣ�������
| A�� | BeCl2 | B�� | CO2 | C�� | HCl | D�� | N2 |
13������һ�����ʣ�������С��0.3g���ı�ȩ����8g����������������Һ��Ӧ��������30.6g��������ʿ����ǣ�������
| A�� | ��ȩ | B�� | ��ȩ | C�� | ��ȩ | D�� | ��ȩ |
3������ʵ������ܴﵽĿ���ǣ�������
| ѡ�� | ʵ�鷽�� | ʵ��Ŀ�� |
| A | �����ס���������������Һ�й���ԭ��� | ��֤�ס��ҵĻ���ǿ�� |
| B | �ö��Ե缫��ⲻͬŨ�ȵ�AgNO3��Cu��NO3��2�Ļ����Һ | ֤������ͭ�������� |
| C | ��������þ������������Һ�м����Ȼ�����Һ | ֤��Ksp[Fe��OH��3]��Ksp[Mg��OH��2] |
| D | �ⶨ0.1mol•L-1Na2X��0.1mol•L-1Na2Y��Һ��pH | ��֤H2X��H2Y���Ե����ǿ�� |
| A�� | A | B�� | B | C�� | C | D�� | D |
7���ᡢ��ξ����ڵ���ʣ����ǵ�ˮ��Һ�д��ڸ���ƽ�⣮
��1����ˮ����ѧ������
��������ʵ��֤����ˮ���������BD������ĸ��ţ���
A����ˮ�ܸ��Ȼ�������Һ��Ӧ��������������
B�������£�0.1mol•L-1��ˮpHΪ11
C����������ֽ�
D�������£�0.1mol•L-1�Ȼ����Һ��pHΪ5
�����з����У�����ʹ��ˮ����̶��������BC������ĸ��ţ���
A��ͨ�백�� B�����������Ȼ�������
C����ˮϡ�� D�����������Ȼ�粒���
��2������ʹ�������ѧ������
��0.1mol•L-1NaOH��Һ�ֱ�ζ������Ϊ20.00mL��Ũ�Ⱦ�Ϊ0.1mol•L-1������ʹ�����Һ���õ��ζ���������ҺpH�����NaOH��Һ������仯�������ζ����ߣ�
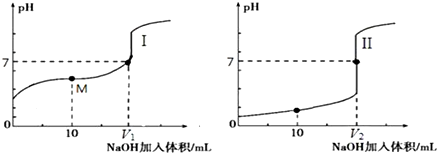
�ٵζ������������I���I����II������
�ڵζ���ʼǰ��������Һ����ˮ�������c��H+��������0.1mol•L-1������Һ��
��V1��V2�Ĺ�ϵ��V1��V2�����������=����������
��M���Ӧ����Һ�У������ӵ����ʵ���Ũ���ɴ�С��˳����c��CH3COO-����c ��Na+����c ��H+����c ��OH-����
��3��Ϊ���о������εij����ܽ�ƽ��ͳ���ת����ijͬѧ�������ʵ�飮
��֪����AgSCN�ǰ�ɫ������
����ͬ�¶��£�Ksp��AgI��=8.3��10?17��Ksp ��AgSCN ��=1.0��10?12��
�ٲ���3������a�dz��ְ�ɫ������
���ó����ܽ�ƽ��ԭ�����Ͳ���4��ʵ������AgSCN��s��?Ag+��aq��+SCN-��aq��������KI����Ϊ�ܽ�ȣ�AgSCN��AgI��Ag+��I-��Ӧ����AgI��ɫ������AgSCN���ܽ�ƽ�������ƶ���
����50mL 0.005mol•L?1��AgNO3��Һ�м���150mL0.005mol•L?1�� KSCN��Һ����Ϻ���Һ��Ag+��Ũ��ԼΪ4��10?10mol•L?1����������Һ����仯��
��1����ˮ����ѧ������
��������ʵ��֤����ˮ���������BD������ĸ��ţ���
A����ˮ�ܸ��Ȼ�������Һ��Ӧ��������������
B�������£�0.1mol•L-1��ˮpHΪ11
C����������ֽ�
D�������£�0.1mol•L-1�Ȼ����Һ��pHΪ5
�����з����У�����ʹ��ˮ����̶��������BC������ĸ��ţ���
A��ͨ�백�� B�����������Ȼ�������
C����ˮϡ�� D�����������Ȼ�粒���
��2������ʹ�������ѧ������
��0.1mol•L-1NaOH��Һ�ֱ�ζ������Ϊ20.00mL��Ũ�Ⱦ�Ϊ0.1mol•L-1������ʹ�����Һ���õ��ζ���������ҺpH�����NaOH��Һ������仯�������ζ����ߣ�

�ٵζ������������I���I����II������
�ڵζ���ʼǰ��������Һ����ˮ�������c��H+��������0.1mol•L-1������Һ��
��V1��V2�Ĺ�ϵ��V1��V2�����������=����������
��M���Ӧ����Һ�У������ӵ����ʵ���Ũ���ɴ�С��˳����c��CH3COO-����c ��Na+����c ��H+����c ��OH-����
��3��Ϊ���о������εij����ܽ�ƽ��ͳ���ת����ijͬѧ�������ʵ�飮
| ����1����2mL 0.005mol•L-1 AgNO3��Һ�м���2mL 0.005mol•L-1KSCN��Һ�����ã� | ���ְ�ɫ������ |
| ����2��ȡ1mL�ϲ���Һ���Թ��У��μ�1��2mol•L-1Fe��NO3��3��Һ�� | ��Һ��Ϊ��ɫ�� |
| ����3������2����Һ�У���������5�� 3mol•L-1AgNO3��Һ�� | ����a���ְ�ɫ��������Һ��ɫ��dz�� |
| ����4������1���µ���Һ�м���5�� 3mol•L-1KI��Һ�� | ���ֻ�ɫ������ |
����ͬ�¶��£�Ksp��AgI��=8.3��10?17��Ksp ��AgSCN ��=1.0��10?12��
�ٲ���3������a�dz��ְ�ɫ������
���ó����ܽ�ƽ��ԭ�����Ͳ���4��ʵ������AgSCN��s��?Ag+��aq��+SCN-��aq��������KI����Ϊ�ܽ�ȣ�AgSCN��AgI��Ag+��I-��Ӧ����AgI��ɫ������AgSCN���ܽ�ƽ�������ƶ���
����50mL 0.005mol•L?1��AgNO3��Һ�м���150mL0.005mol•L?1�� KSCN��Һ����Ϻ���Һ��Ag+��Ũ��ԼΪ4��10?10mol•L?1����������Һ����仯��
8����֪����Ϊ��Ԫ��������£���ijŨ�ȵIJ�����Һ����μ���һ����Ũ�ȵ�KOH��Һ��������Һ��H2C2O4��HC2O4-��C2O42-�����������ʵ����������ģ�����ҺpH�Ĺ�ϵ��ͼ��ʾ��������˵���в���ȷ���ǣ�������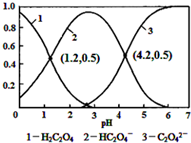

| A�� | pH=1.2��Һ�У�c��K+��+c��H+���Tc��OH-��+c��H2C2O4�� | |
| B�� | pH=2.7��Һ�У�$\frac{{c}^{2}��H{C}_{2}{O}_{4}^{-}��}{c��{H}_{2}{C}_{2}{O}_{4}��}$��c��C2O42-��=1000 | |
| C�� | ����ͬ���ʵ���KHC2O4��K2C2O4������ȫ����ˮ���û��Һ��pHΪ4.2 | |
| D�� | ��pH=1.2����Һ�м�KOH��Һ��pH������4.2�Ĺ�����ˮ�ĵ����һ������ |