��Ŀ����
12��CO2��ת�����л���ʵ��̼ѭ����CO2��CH2OH��HCOOH��1�������ӷ���ʽ��ʾHCOONa��Һ�ʼ��Ե�ԭ��HCOO-+H2O=HCOOH+OH-��
��2�������£���0.2mol•L-1��HCOOH��0.1mol•L-1��NaOH��Һ�������ϣ�������Һ��pH��7��˵��HCOOH�ĵ���̶ȴ���HCOONa��ˮ��̶ȣ�����ڡ���С�ڡ������û����Һ������Ũ���ɴ�С��˳����C��HCOO-����C��Na+����C��H+����C��OH-����
���� ��1��HCOONa��Һˮ��ʼ��ԣ�
��2����0.2mol•L-1��HCOOH��0.1mol•L-1��NaOH��Һ�������ϣ���Ӧ�����ɵ����ʵ�����HCOOH��HCOONa�����ݻ����Һ��������жϣ�
��� �⣺��1��HCOONa��Һˮ��ʼ��ԣ�ˮ�ⷽ��ʽΪ��HCOO-+H2O=HCOOH+OH-��
�ʴ�Ϊ��HCOO-+H2O=HCOOH+OH-��
��2��������ʵ����Ǽ�����ʵ�����2����������һԪ�ģ���Ӧ�����ɵ����ʵ�����HCOOH��HCOONa��HCOOH�����������ӣ�HCOONaˮ���������������ӣ���Һ�����ԣ�˵���Ե���Ϊ������HCOOH�ĵ���̶ȴ���HCOONa��ˮ��̶ȣ�HCOOH��HCOONa�ʹ��ᶼ�������ɴ�������ҵ������ˮ�⣬�ʸû����Һ������Ũ���ɴ�С��˳���ǣ�C��HCOO-����C��Na+����C��H+����C��OH-��
�ʴ�Ϊ�����ڣ�C��HCOO-����C��Na+����C��H+����C��OH-����
���� ���⿼�����ε�ˮ��ԭ����Ӧ�á���ʽ�ε�������ӵĵ�����ˮ��̶ȵıȽϵȣ���Ŀ�ѶȲ������ڻ���֪ʶ�Ŀ��飬ע������ε�ˮ�����ӷ���ʽ����д��

��ϰ��ϵ�д�
�����Ŀ
3��ʵ�鱨���У��������ݺ����������������ǣ�������
| A�� | ��������ƽ��ȡ11.7gNaCl | |
| B�� | ��50mL��Ͳ��ȡ21.48mLϡ���� | |
| C�� | �ü�ʽ�ζ�����ȡ25.03mLH2SO4��Һ | |
| D�� | ��pH��ֽ�ⶨHNO3��Һ��pH=3.7 |
20�����������г����ı仯�У������ڻ�ѧ�仯��һ���ǣ�������
| A�� | ��ҵ�������������� | B�� | ʯ���ۻ����ɱ����� | ||
| C�� | ��ʳ��ơ��̻�ȼ�� | D�� | ���ͻӷ���������ɢ |
7�������б仯�У������ڻ�ѧ�仯���ǣ�������
| A�� | SO2ʹƷ����Һ��ɫ | B�� | ˫��ˮʹ��ɫ������ɫ | ||
| C�� | ����̿ʹ��īˮ��ɫ | D�� | O3ʹijЩȾ����ɫ |
17�����������ʵ���Ȳ4.16g��H2�ӳ����ɱ�������������ȥ4.48L����״����H2�����������ʲ������ǣ�������
| A�� | ��ϩ | B�� | ��Ȳ | C�� | ����ϩ | D�� | ���� |
4�����100ml��c��H+��=0.40mol/L��������Һ������·��ͨ��0.04mol����ʱ�������������������������ǣ�������
| A�� | 0.10mol/L Ag+ | B�� | 0.20mol/L Zn2+ | C�� | 0.20mol/L Cu2+ | D�� | 0.20mol/L Pb2+ |
 ��
��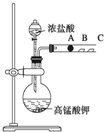 ij�о���ѧϰС�������һ��ʵ����̽��Ԫ�������ɣ���ͬѧ�������ͼװ������֤±��Ԫ�����ʵĵݱ���ɣ�A��B��C�����ֱ���մ��NaBr��Һ������ʪ��ĵ���KI��ֽ��ʪ���ֽ����֪������Ũ�������������ܷ�Ӧ����������
ij�о���ѧϰС�������һ��ʵ����̽��Ԫ�������ɣ���ͬѧ�������ͼװ������֤±��Ԫ�����ʵĵݱ���ɣ�A��B��C�����ֱ���մ��NaBr��Һ������ʪ��ĵ���KI��ֽ��ʪ���ֽ����֪������Ũ�������������ܷ�Ӧ����������