��Ŀ����
2012�����Ṥҵ��Ⱦ���ŷű��ж���������β���еĺ������ó���0.05mg/m3���ɼ����Ṥҵ������Ҫ���Ǿ���Ч�棬ҲҪ�������Ч�森
��1��������������������Ĺ����У���֮�ص��豸��
a�������� b���Ӵ��� c������¯ d������¯
��2��Ϊ�����SO2ת��ΪSO3��������������ҵ�ϳ�ʹ���ʵ������Ŀ����������ӽӴ��ҵ���������ijɷ��� ��
��3�������Ṥҵ�У�����ŨH2SO4������ˮ����SO3����Ϊ �� Ϊ���������᳧β���ŷŶԻ�����Ⱦ�����ð�ˮ����SO2����ѧ����ʽΪ ��
��4����������ԭ�����ɷ֣����������Ϊ��SO2 8%��O2 11%��N2 82%��ѹǿ���¶ȶ�SO2ת���ʵ�Ӱ�����£�
��ҵ�ϣ�����������������̲��õ��dz�ѹ�����Ǹ�ѹ����Ҫԭ���� �������ϱ����ݿ�֪����������Ĵ������Ƿ��ȷ�Ӧ�������� ��
��5���Ӵ���������Ĺ����У���֪����Ӵ��ҵ������������ǣ�SO2ռ8%��N2ռ81%��O2ռ11%����һ���¶��£���Ӧ�ﵽƽ����������Ϊԭ����96.5%����SO2ת��ΪSO3�İٷ���Ϊ �����¶������µ�ƽ�ⳣ���� ��
��1��������������������Ĺ����У���֮�ص��豸��
a�������� b���Ӵ��� c������¯ d������¯
��2��Ϊ�����SO2ת��ΪSO3��������������ҵ�ϳ�ʹ���ʵ������Ŀ����������ӽӴ��ҵ���������ijɷ���
��3�������Ṥҵ�У�����ŨH2SO4������ˮ����SO3����Ϊ
��4����������ԭ�����ɷ֣����������Ϊ��SO2 8%��O2 11%��N2 82%��ѹǿ���¶ȶ�SO2ת���ʵ�Ӱ�����£�
 |
0.1 | 0.5 | 1 | 10 |
| 400 | 99.2 | 99.6 | 99.7 | 99.9 |
| 500 | 93.5 | 96.9 | 97.8 | 99.3 |
| 600 | 73.7 | 85.8 | 89.5 | 96.4 |
��5���Ӵ���������Ĺ����У���֪����Ӵ��ҵ������������ǣ�SO2ռ8%��N2ռ81%��O2ռ11%����һ���¶��£���Ӧ�ﵽƽ����������Ϊԭ����96.5%����SO2ת��ΪSO3�İٷ���Ϊ
���㣺��ѧƽ���Ӱ������,��ѧƽ��ĵ�������,��ѧƽ��ļ���,���������������Ⱦ������
ר�⣺��ѧƽ��ר��
��������1����ҵ������Ĺ�����������ʯ�ڷ���¯��ȼ�����ɶ��������������ڽӴ���������Ϊ�������������������������б�Ũ���������������
��2����������������Ϊ���������ǿ��淴Ӧ��
��3��������������ˮ������������Ӧ�������γ�������ֹ��������������գ�
��4���������ݽ����жϣ���ѹʱSO2��ת�����Ѿ��ܴ�
��5���ٶ���Ӧǰ��������Ϊ100����SO2�����Ϊ8��N2�����Ϊ81��O2�����Ϊ11��������������Ӧ�����������Ϊx����Ӧƽ�������������Ϊ8-2x+11-x+2x+81=96.5�����x=3.5���Դ˼���SO2ת����K��
��2����������������Ϊ���������ǿ��淴Ӧ��
��3��������������ˮ������������Ӧ�������γ�������ֹ��������������գ�
��4���������ݽ����жϣ���ѹʱSO2��ת�����Ѿ��ܴ�
��5���ٶ���Ӧǰ��������Ϊ100����SO2�����Ϊ8��N2�����Ϊ81��O2�����Ϊ11��������������Ӧ�����������Ϊx����Ӧƽ�������������Ϊ8-2x+11-x+2x+81=96.5�����x=3.5���Դ˼���SO2ת����K��
���
�⣺��1����ҵ������Ĺ�����������ʯ�ڷ���¯��ȼ�����ɶ��������������ڽӴ���������Ϊ�������������������������б�Ũ���������������ᣬ����֮�ص��豸������¯��
�ʴ�Ϊ��d��
��2����������������Ϊ���������ǿ��淴Ӧ����˴ӽӴ����е�����������SO3��O2��SO2��N2��
�ʴ�Ϊ��SO3��O2��SO2��N2��
��3����������SO3�����ˮ���գ�������Ӧ��SO3+H2O�TH2SO4���÷�ӦΪ���ȷ�Ӧ���ų����������������γɣ���������������ˮ֮�䣬�谭ˮ��������������գ���Ũ����ķе�ߣ����������������γ�������ͬʱ��������������Ũ���ᣬ���Թ�ҵ�ϴ���������������Ũ����������Һ�����յõ������̡����ᣮ���������백����Ӧ����������泥�����ʽΪ��SO2+2NH3+H2O�T��NH4��2SO3��
�ʴ�Ϊ����ˮ�������γ��������谭SO3�����գ�SO2+2NH3+H2O�T��NH4��2SO3��
��4���ɱ������ݿ�֪����ѹʱSO2��ת�����Ѿ��ܴ�����ѹǿ�����Ӷ����ɱ����豸�ɱ����ɱ������ݿ�֪��ѹǿһ��ʱ�������¶ȵ����ߣ�ת���ʼ�С��
�ʴ�Ϊ����ѹʱSO2��ת�����Ѿ��ܴ�����ѹǿ�����Ӷ����ɱ����豸�ɱ���ѹǿһ��ʱ���¶�����SO2ת�����½���
��2���������Ϊ100����SO2�����Ϊ8��N2�����Ϊ81��O2�����Ϊ11��������������Ӧ��������������Ϊx����
2SO2 +O2=2SO3
��ʼ 8 11 0
ת�� x 0.5x x
ƽ��8-x 11-0.5x x
=96.5%��x=7
ת����=
��100%=87.5%��
K=
=6.53��
�ʴ�Ϊ��87.5%��6.53��
�ʴ�Ϊ��d��
��2����������������Ϊ���������ǿ��淴Ӧ����˴ӽӴ����е�����������SO3��O2��SO2��N2��
�ʴ�Ϊ��SO3��O2��SO2��N2��
��3����������SO3�����ˮ���գ�������Ӧ��SO3+H2O�TH2SO4���÷�ӦΪ���ȷ�Ӧ���ų����������������γɣ���������������ˮ֮�䣬�谭ˮ��������������գ���Ũ����ķе�ߣ����������������γ�������ͬʱ��������������Ũ���ᣬ���Թ�ҵ�ϴ���������������Ũ����������Һ�����յõ������̡����ᣮ���������백����Ӧ����������泥�����ʽΪ��SO2+2NH3+H2O�T��NH4��2SO3��
�ʴ�Ϊ����ˮ�������γ��������谭SO3�����գ�SO2+2NH3+H2O�T��NH4��2SO3��
��4���ɱ������ݿ�֪����ѹʱSO2��ת�����Ѿ��ܴ�����ѹǿ�����Ӷ����ɱ����豸�ɱ����ɱ������ݿ�֪��ѹǿһ��ʱ�������¶ȵ����ߣ�ת���ʼ�С��
�ʴ�Ϊ����ѹʱSO2��ת�����Ѿ��ܴ�����ѹǿ�����Ӷ����ɱ����豸�ɱ���ѹǿһ��ʱ���¶�����SO2ת�����½���
��2���������Ϊ100����SO2�����Ϊ8��N2�����Ϊ81��O2�����Ϊ11��������������Ӧ��������������Ϊx����
2SO2 +O2=2SO3
��ʼ 8 11 0
ת�� x 0.5x x
ƽ��8-x 11-0.5x x
| 8-x+11-0.5x+x+81 |
| 100 |
ת����=
| 7 |
| 8 |
K=
| 7��7 |
| 1��1��7.5 |
�ʴ�Ϊ��87.5%��6.53��
���������⿼�鹤ҵ�Ӵ����������ԭ������ؼ��㣬�Ѷ��еȣ�

��ϰ��ϵ�д�
 �����Ƹ���ʦ����ϵ�д�
�����Ƹ���ʦ����ϵ�д� ��ͨ����ͬ����ϰ��ϵ�д�
��ͨ����ͬ����ϰ��ϵ�д� ����С����ͬ������ϵ�д�
����С����ͬ������ϵ�д�
�����Ŀ
Li-Al/FeS�����һ�����ڿ����ĵ綯���õ�أ��õ�ط�ӦʽΪ��2Al+6Li++3FeS�T2Al3++3Li2S+3Fe�����йظõ�ص�����˵���в���ȷ���ǣ�������
| A���õ��������ڵ綯���㷺ʹ�õ�Ǧ���ؿ��Լ����ؽ�������Ⱦ |
| B���õ�طŵ�ʱ��Һ��Li+�������������� |
| C���õ�صĸ����缫��ӦʽΪAl-3e-=Al3+ |
| D�����ʱ�����������ĵ缫��ӦʽΪLi2S+Fe-2e-=2Li++FeS |
NAΪ����٤��������ֵ������������ȷ���ǣ�������
| A��1.0L 1.0mo1?L-1��NaAlO2ˮ��Һ�к��е���ԭ����Ϊ2NA |
| B��12gʯīϩ������ʯī���к�����Ԫ���ĸ���Ϊ0.5NA |
| C��25��ʱpH=13��NaOH��Һ�к���OH-����ĿΪ0.1NA |
| D��1mol���ǻ���1moL������������������������Ϊ9NA |
��NA��ʾ�����ӵ�����������˵����ȷ���ǣ�������
| A��18g D2O���е�����Ϊ9NA |
| B����״���£�11.2L�����к��еķ�����ĿΪ0.5NA |
| C��78g Na2O2�к��е���������Ϊ4NA |
| D��1L 1mol?L-1 Na2CO3��Һ�У���Һ��CO32-��������NA |


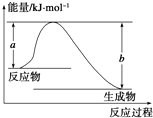 �Ͽ�1mol AB��g�������еĻ�ѧ��ʹ��ֱ�������̬Aԭ�Ӻ���̬Bԭ�������յ�������ΪA-B���ļ��ܣ��±��г���һЩ��ѧ���ļ���E��
�Ͽ�1mol AB��g�������еĻ�ѧ��ʹ��ֱ�������̬Aԭ�Ӻ���̬Bԭ�������յ�������ΪA-B���ļ��ܣ��±��г���һЩ��ѧ���ļ���E�� ��10mL NaOH��Һ��������ͨ��һ������CO2���õ���Na2CO3��NaHCO3�Ļ����Һ����������Һ����μ���0.1mol/L�����ᣬ�ӱ���ʹ���ַ�Ӧ������CO2������������״�����������������֮��Ĺ�ϵ��ͼ��ʾ��
��10mL NaOH��Һ��������ͨ��һ������CO2���õ���Na2CO3��NaHCO3�Ļ����Һ����������Һ����μ���0.1mol/L�����ᣬ�ӱ���ʹ���ַ�Ӧ������CO2������������״�����������������֮��Ĺ�ϵ��ͼ��ʾ�� S2Cl2�����������л����Ȼ����Լ���ij�о���ѧϰС������ʵ���ҳ����Լ��������ϳ�S2Cl2��
S2Cl2�����������л����Ȼ����Լ���ij�о���ѧϰС������ʵ���ҳ����Լ��������ϳ�S2Cl2��
 ��������������ˮʾ��ͼ��ͼ��ʾ�����������������ӽ���Ĥ������������������ͨ����
��������������ˮʾ��ͼ��ͼ��ʾ�����������������ӽ���Ĥ������������������ͨ����