��Ŀ����
1��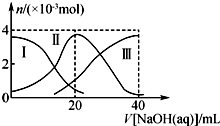 �����£���20mL 0.2mol•L-1H2A��Һ�еμ�0.2mol•L-1 NaOH��Һ���й��������ʵ����仯����ͼ�������H2A�������HA-�������A2-��������˵����ȷ���ǣ�������
�����£���20mL 0.2mol•L-1H2A��Һ�еμ�0.2mol•L-1 NaOH��Һ���й��������ʵ����仯����ͼ�������H2A�������HA-�������A2-��������˵����ȷ���ǣ�������| A�� | H2A��ˮ�еĵ��뷽��ʽΪH2A�TH++HA-��HA-?H++A2- | |
| B�� | ��NaHA��Һ�м�ˮ��HA-�ĵ����������Һ��pH��С | |
| C�� | V��NaOH��=20 mLʱ����Һ������Ũ�ȴ�С��ϵ��c��Na+����c��HA-����c��H+����c��A2-����c��OH-�� | |
| D�� | V��NaOH��=30 mLʱ����Һ�д������¹�ϵ��2c��H+��-2c��OH-��=c��A2-��-3c��H2A��-c��HA-�� |
���� A��0.2mol•L-1H2A��Һc��H+����0.4mol/L����H2A����Һ�в��ֵ��룬Ϊ���
B����NaHA��Һ�м�ˮ��HA-�ĵ��������c��H+����С��
C������ͼ��֪����V��NaOH��=20ʱ��������ӦΪNaOH+H2A=NaHA+H2O����Һ��ҪΪNaHA������Ϊ������Һ�����ԣ�
D����V��NaOH��=30mLʱ��������ӦΪNaOH+H2A=NaHA+H2O��NaHA+NaOH=Na2A+H2O����Һ��ҪΪ����������NaHA��Na2A�Ļ����Һ�����ݵ���غ�������غ�
��� �⣺A��0.2mol•L-1H2A��Һc��H+����0.4mol/L����H2A����Һ�в��ֵ��룬Ϊ���ᣬH2A��ˮ�еĵ��뷽��ʽ�ǣ�H2A?H++HA-��HA-?H++A2-����A����
B����NaHA��Һ�м�ˮ��HA-�ĵ��������������Һ�������̶ȴ��������ӵ����ʵ�������̶ȣ�����c��H+����С����ҺpH����B����
C����V��NaOH��=20 mLʱ��������ӦΪNaOH+H2A=NaHA+H2O����Һ��ҪΪNaHA��HA-�������ˮ�⣬��Һ�����ԣ���c��Na+����c��HA-����c��H+����c��A2-����c��OH-������C��ȷ��
D����V��NaOH��=30mLʱ��������ӦΪNaOH+H2A=NaHA+H2O��NaHA+NaOH=Na2A+H2O����Һ��ҪΪ����������NaHA��Na2A�Ļ����Һ�����ݵ���غ�ã�c��Na+��+c��H+��=c��HA-��+2c��A2-��+c��OH-���٣������غ��֪��3c��HA-��+3c��A2-��+3c��H2A��=2c��Na+���ڣ��١�2+�ڵã�2c��H+��-2c��OH-��=c��A2-��-3c��H2A��-c��HA-������D��ȷ��
��ѡCD��
���� ���⿼���������Һ�����жϣ�������ѧ���ķ��������Ŀ��飬��ȷͼ���������ʱ��Һ�е������ǽ����Ĺؼ���ץסͼ����з������ɣ���Ŀ�Ѷ��еȣ�

| A�� | ��ϩ�Ľṹ��ʽΪCH2CH2 | B�� | CO2���ӵĽṹʽ��O=C=O | ||
| C�� | ��������ĵ���ʽ��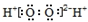 | D�� | ���Ȼ�̼���ӱ���ģ�ͣ� |
| A�� | ${\;}_{34}^{78}$Se��${\;}_{34}^{80}$Se��Ϊͬ�������� | |
| B�� | ${\;}_{34}^{78}$Se��${\;}_{34}^{80}$Se��Ϊͬλ�� | |
| C�� | ${\;}_{34}^{78}$Se��${\;}_{34}^{80}$Se�������ʺͻ�ѧ���ʾ���ͬ | |
| D�� | ${\;}_{34}^{78}$Se��${\;}_{34}^{80}$Se������34������ |
| A�� | H3BO3�����Ա�H2CO3��ǿ | |
| B�� | Mg��OH��2�ļ��Ա�Be��OH��2��ǿ | |
| C�� | C��N��Oԭ�Ӱ뾶�������� | |
| D�� | ��M+��R2-�ĺ�����Ӳ�ṹ��ͬ����ԭ��������R��M |
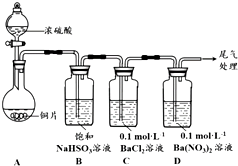 ������ͬѧ�ֱ�Ժ�+4����Ԫ�ص��������ʽ�����̽����
������ͬѧ�ֱ�Ժ�+4����Ԫ�ص��������ʽ�����̽������1��������ͼװ�ý���ʵ�飨�������Ѽ��飬���Ⱥͼг�װ������ȥ����ʵ�����һ��ʱ���C��D�ж��������Եİ�ɫ����������
���ΪBaSO4��
��A�з�Ӧ�Ļ�ѧ����ʽ��Cu+2H2SO4��Ũ��$\frac{\underline{\;\;��\;\;}}{\;}$CuSO4+SO2��+2H2O��
��Ϊ̽��SO2��D���������ķ�Ӧ����һ��ʵ�鷢�֣����ְ�ɫ�����Ĺ����У�D��Һ��NO3-Ũ�ȼ������䣮�ݴ˵ó����ۣ�D�г��ְ�ɫ��������Ҫԭ�������������£���+4����Ԫ�����ʣ�SO2��H2SO3����O2��������SO42-��
��2����������ʵ��Ժ�+4����Ԫ�ص��������ʼ�������̽����
| ��� | ʵ����� | ʵ������ |
| 1 | ȡ0.3g ����Na2SO3���壬�����м���10mL 2mol•L-1 ���ᣬ�ٵ���4��BaCl2��Һ | ������ɫ���ݣ�����BaCl2��Һ��ʼ������4min����Һ����� |
| 2 | ȡ0.3g ����Na2SO3���壬�����м���10mL 2mol•L-1 HNO3���ٵ���4��BaCl2��Һ | ������ɫ���ݣ�����BaCl2��Һ��ʼ������2h����Һ����� |
| 3 | ȡ0.3g ����Na2SO3���壬�����м���10mL ŨHNO3���ٵ���4��BaCl2��Һ | ��������ɫ���壻����BaCl2��Һ����Һ��������������ɫ���� |
����ʵ��1��2��3�Աȣ����Եõ����ۣ���+4����Ԫ�����ʿɱ�O2��ŨHNO3������
����ͨ���������Ϸ��֣�Na+��ʵ��1��2�г��ֻ��ǵ�ʱ����Ӱ�죬���ǽ�һ��̽��Cl-��NO3-�����Ӱ�죺
| ��� | ʵ����� | ʵ������ |
| 4 | ȡ0.3g����Na2SO3��1.17gNaCl������������м���10mL 2mol•L-1 HNO3���ٵ���4��BaCl2��Һ | ������ɫ���ݣ�����BaCl2��Һ��ʼ������20min����Һ����� |
ii��ʵ��1��4�Աȣ��һ�����ۣ�NO3-�Ĵ��ڿ��Լ�����Һ��+4����Ԫ�ص�������
��ͨ������ʵ�飬��ͬѧ��Ϊ��ȷ��ij��Һ�к���SO42-��ʵ�鷽����ȡ����Һ���������ȵμ�bd������ĸ��ţ���
a.2mol•L-1���ᣬ�ٵμ�BaCl2��Һ��һ��ʱ�����ְ�ɫ����
b.2mol•L-1���ᣬ�ٵμ�BaCl2��Һ���������ְ�ɫ����
c.2mol•L-1���ᣬ�ٵμ�BaCl2��Һ��һ��ʱ�����ְ�ɫ����
d.2mol•L-1���ᣬ�ٵμ�BaCl2��Һ���������ְ�ɫ������
| A�� | �����ڵ�����У�O2-�ɸ����������� | |
| B�� | ͨ�������һ�����������缫��ӦΪ��O2+2H2O+4e-�T4OH- | |
| C�� | ͨ������һ���Ǹ������缫��ӦΪ��CH4+8e-+4O2-�TCO2+2H2O | |
| D�� | ����·��ͨ��amol����ʱ�������������ɱ�״����CO2����2.8aL |
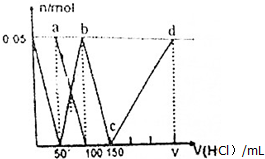
| A�� | a����Һ�У�c��HCO${\;}_{3}^{-}$��+c��H2CO3��+c��H+��=c��OH-�� | |
| B�� | ������Һ�еμ�75nL����ʱ����Һ�в�������Ũ�ȴ�С˳��Ϊ��c��Na+����c��Cl-����c��CO${\;}_{3}^{2-}$����c��OH-����c��H+�� | |
| C�� | b����Һ�У�c��Cl-��+c��HCO3-��+c��H2CO3��+c��CO${\;}_{3}^{2-}$��=1.5mol•L-1 | |
| D�� | c��d�Ĺ�����ˮ�ĵ���̶���С |
| A�� |  | B�� |  | C�� |  | D�� |  |
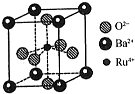 �ϳɰ��ǻ�ѧ��ѧ��������ᷢչ������������ĵ���֮һ���ϳɰ���ҵ����ԭ�������Ʊ������������ĺϳɼ����ֻ��������ȣ�
�ϳɰ��ǻ�ѧ��ѧ��������ᷢչ������������ĵ���֮һ���ϳɰ���ҵ����ԭ�������Ʊ������������ĺϳɼ����ֻ��������ȣ�