��Ŀ����
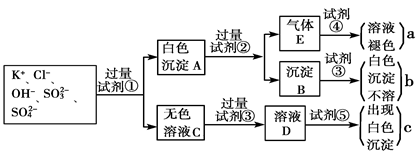
��13�֣�ijǿ������ҺX���ܺ���Ba2+��A13+��NH4+��Fe2+��Fe3+��CO32-��SO32-��SO42-��C1-��NO3-�е�һ�ֻ��֣�ȡ����Һ��������ʵ�飬ʵ��������£�����������Ϣ���ش��������⣺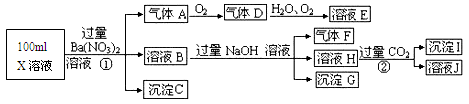
��1������FΪ____________��
��2�����������У���ҺX�г�H+��϶����е�������_______������ȷ���Ƿ��е�������____��
��3��д������A�����ӷ���ʽ��_________________��
��4��ͨ����������KClO�ڼ�������������G���Ʊ�һ�����͡���Ч�����ˮ������K2FeO4����д���Ʊ������е����ӷ���ʽ__________________��
��1��NH3 ��2��Al3+��NH4+��Fe2+��SO42-��Fe3+��Cl- ��3��3Fe2++NO3-+4H+��3Fe3++NO��+2H2O
��4��3ClO-+2Fe��OH��3+4OH-�T3Cl-+2FeO42-+5H2O
�������������ǿ������Һ��һ���������CO32-��SO32-���ӣ�����������ᱵ���ɳ�������ó���ΪBaSO4������˵����Һ�к���SO42-���ӣ���������A��A������������D��E����AΪNO��DΪNO2��EΪHNO3��˵����Һ�к��л�ԭ�����ӣ�һ��ΪFe2+���ӣ���ҺB�м������NaOH��Һ����������F����FΪNH3��˵����Һ�к���NH4+���ӣ���ҺH������CO2���壬���ɳ���I����IΪAl��OH��3��HΪNaAlO2��˵����Һ�к���Al3+���ӣ���Һ�к���Fe2+���ӣ���һ������NO3-���ӣ�����SO42-���Ӿ�һ������Ba2+���ӣ�����ȷ���Ƿ��е�����Fe3+��Cl-����
��1��������������֪������FΪNH3��
��2��������������֪��һ������Al3+��NH4+��Fe2+��SO42-�����ܺ�Fe3+��Cl-��
��3��AΪNO������A�����ӷ���ʽΪ3Fe2++NO3-+4H+��3Fe3++NO��+2H2O��
��4��Fe2+���ӱ�����ΪFe3+���ӣ�����NaOH��Һ������G��Fe��OH��3���ʹ����ᷴӦ��ø�����صķ���ʽΪ3ClO-+2Fe��OH��3+4OH-�T3Cl-+2FeO42-+5H2O��
���㣺�������ӵļ��鼰�ƶ�

�����£����и����������ض���Һ��һ���ܴ����������
| A��pH=12�ij������Һ�У�K����Na����MnO4����SO42�� |
| B��c(Al3+) ="0.1" mol��L��1����Һ�У�Na����Cl����HCO3����SO42�� |
| C�����ȳʺ�ɫ����Һ�У�NH4����Ba2����AlO2����Cl�� |
| D�������������ۺ��ܲ�����������Һ�У�NH4����Fe2����NO3����SO42�� |
���з�Ӧ�����ӷ���ʽ��ȷ����
A������������ˮ�Ʊ������Cl2+H2O 2H++Cl-+ClO- 2H++Cl-+ClO- |
| B������CO2ͨ�뱽������Һ�У�2C6H5O-+CO2+H2O=2C6H5OH+CO32- |
| C��Na2O2��H2O��ϣ�2Na2O2+2H2O=4Na++4OH-+O2�� |
D����NH4HCO3��Һ�мӹ�����NaOH��Һ�����ȣ�NH4++OH- NH3��+H2O NH3��+H2O |
����Һ����ˮ���������C (OH��)��1��10��14mol��L��1���������������Һ��һ�����Դ���������������ǣ�
| A��Al3�� Na��NO3�� Cl�� | B��K�� Na��Cl�� NO3�� |
| C��K�� Na�� Cl��AlO2�� | D��K�� NH4�� SO42��NO3�� |
�������ӷ���ʽ����д��ȷ���ǣ� ��
| A������ϡ���ᷴӦ��2Fe + 6H+ ��2Fe 3+ +3H 2�� |
| B��NaHCO3��Һ��NaOH��Һ��Ӧ�� OH�D + HCO3�D�� CO32�D + H2O |
| C���ƺ���ˮ��Ӧ Na��2H2O��Na+��2OH-��H2�� |
| D���Ȼ�����Һ�м�������İ�ˮ Al3+ + 4NH3��H2O ��AlO2�� + 4NH4++ 2H2O |
��10�֣�ij����С����Ƴ����ù�ҵ����(l0%)���ѽ�ij����������ͭп��ķ�����ʵ�ַ����ۺ����ã�����ͼ��ʾ��
��֪�������ӿ�ʼ��������ȫ����ʱ��pH���±���ʾ��
��ش��������⣺
��1������ͭп���к���������CuS��ZnS����H2SO4��������ZnS�����ܽ��CuS
���ܣ�����ͬ�¶��£�Ksp(CuS)______Ksp(ZnS)��ѡ�>����<����=������
��2������A��ʹ�����������е� ��
| A��KMnO4 | B��O2 | C��H2O2 | D��Cl2 |
��4������B��ֱ���������ʣ���B�Ļ�ѧʽ��________��
��5��������õ���Fe(OH)3����KClO��Һ�ڼ��Ի����½��������õ�һ�ָ�Ч�Ķ��ˮ����������K2FeO4��д���÷�Ӧ�����ӷ���ʽ__________��
�ס��ҡ��������ֲ�����ͬ���ӵĿ�����ǿ����ʡ������������������±���ʾ��
| ������ | NH4+��Na����Mg2�� |
| ������ | OH����NO3����SO42�� |
ȡ�����������ֻ��������Ƴ���ͬ�������Һ�������ʵ����ʵ���Ũ�ȣ�c(��)��c(��)��c(��)��
(1)����________����AlCl3��Һ����μ������Һ���۲쵽��������________����Ӧ�����ӷ���ʽΪ________��
(2)����______�����ʵ��ȷ���ҵ��������________(������ȷ��������ÿ�)��